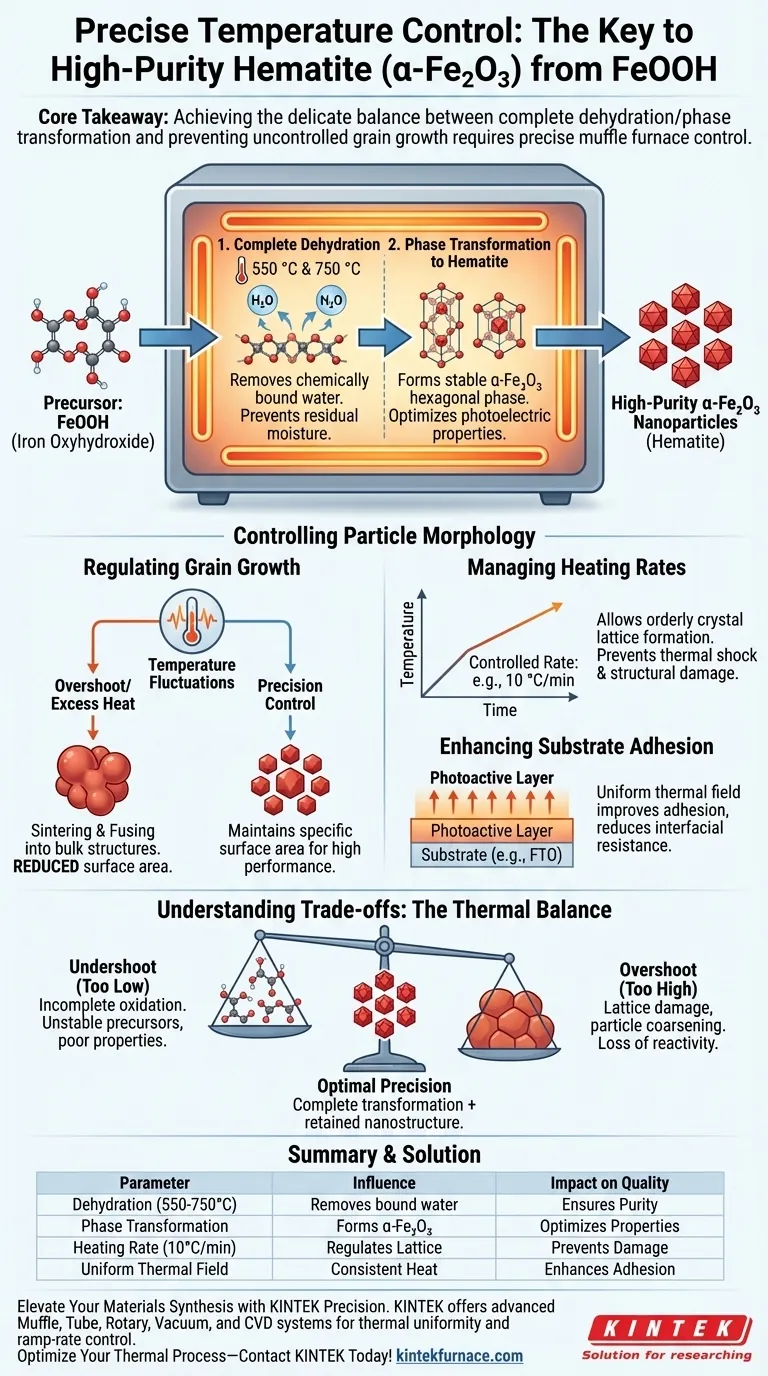
Precise temperature control in a muffle furnace is the defining factor in achieving high-purity hematite ($\alpha$-Fe$_2$O$_3$) without compromising particle structure. It directly regulates the dehydration of FeOOH and manages the critical crystalline phase transformation, ensuring the material achieves the correct state while preventing uncontrolled grain growth.
Core Takeaway Success in converting FeOOH to Fe$_2$O$_3$ requires a delicate balance: the temperature must be high enough to ensure complete dehydration and phase transformation, but stable enough to prevent the nanoparticles from fusing into larger, less effective grains.

Driving Phase Purity and Crystallinity
The primary function of the muffle furnace in this process is to facilitate the complete chemical conversion of the precursor material.
Ensuring Complete Dehydration
The conversion process begins with the removal of chemically bound water from the iron oxyhydroxide (FeOOH).
Specific multi-stage sintering temperatures, often set at benchmarks such as 550 °C and 750 °C, are required to drive this reaction to completion. Without precise adherence to these thermal setpoints, residual moisture or intermediate phases may persist, compromising the material's purity.
Targeting the Hematite Phase
The ultimate goal is the formation of the $\alpha$-Fe$_2$O$_3$ (hematite) crystal phase.
Precise temperature uniformity ensures that the thermal field surrounding the sample is consistent. This facilitates the transformation of amorphous or unstable precursors into the stable hexagonal phase hematite crystals necessary for photoelectric applications.
Controlling Particle Morphology
Beyond chemical composition, the physical structure of the resulting oxide is strictly determined by how heat is applied.
Regulating Grain Growth
Temperature fluctuations or excessive heat are the primary causes of excessive grain growth.
If the temperature overshoots, the diffusion rate of atoms increases, causing nanoparticles to sinter and fuse into larger, bulk-like structures. Precision control maintains the specific surface area required for high-performance nanomaterials.
Managing Heating Rates
The rate at which temperature increases is as critical as the final dwell temperature.
A controlled heating rate, such as 10 °C/min, allows for orderly crystal lattice formation. This prevents structural damage that can occur from thermal shock or uneven expansion during the ramp-up phase.
Enhancing Substrate Adhesion
For applications where the oxide is grown on a substrate (like FTO), the thermal treatment dictates mechanical integrity.
A uniform thermal field enhances the adhesion between the photoactive layer and the substrate. This reduces interfacial resistance, which is vital for efficient electrical contact.
Understanding the Trade-offs
When defining your thermal profile, you are balancing reaction kinetics against structural preservation.
The Consequence of Undershooting
If the temperature is too low or the heating is non-uniform, the oxidation and phase transformation will be incomplete.
This leaves behind unstable precursors or amorphous phases that lack the desired photoelectric or magnetic properties.
The Risk of Overshooting
If the temperature is too high or fluctuates upward, you risk lattice damage and "coarsening" of the particles.
While the chemical conversion may be complete, the functional performance drops because the fine nanoparticle structure—critical for surface reactivity—is lost to sintering.
Making the Right Choice for Your Goal
To optimize the conversion of FeOOH to Fe$_2$O$_3$, tailor your furnace settings to your specific performance metrics.
- If your primary focus is Phase Purity: Ensure your furnace can hold strict multi-stage dwell times at 550 °C and 750 °C to guarantee complete transformation to $\alpha$-Fe$_2$O$_3$.
- If your primary focus is Nanoparticle Size: Prioritize a furnace with excellent ramp-rate control (e.g., 10 °C/min) and stability to prevent temperature spikes that trigger grain growth.
Ultimately, the quality of your final hematite product is less about the maximum temperature reached and more about the precision and uniformity of the thermal path taken to get there.
Summary Table:
| Parameter | Influence on Process | Impact on Quality |
|---|---|---|
| Dehydration (550°C - 750°C) | Removes bound water from FeOOH | Prevents residual moisture & ensures purity |
| Phase Transformation | Formation of $\alpha$-Fe$_2$O$_3$ (hematite) | Optimizes photoelectric and magnetic properties |
| Heating Rate (e.g., 10 °C/min) | Regulates crystal lattice formation | Prevents thermal shock and structural damage |
| Uniform Thermal Field | Consistent heat distribution | Enhances substrate adhesion and reduces resistance |
| Grain Control | Limits atomic diffusion rates | Maintains high surface area for nanomaterials |
Elevate Your Materials Synthesis with KINTEK Precision
Don't let temperature fluctuations compromise your high-purity hematite. Backed by expert R&D and manufacturing, KINTEK offers advanced Muffle, Tube, Rotary, Vacuum, and CVD systems designed to deliver the thermal uniformity and ramp-rate control essential for sensitive phase transformations. Whether you need standard lab high-temp furnaces or a fully customizable solution for your unique research needs, our team is here to help you achieve consistent, high-performance results.
Optimize Your Thermal Process—Contact KINTEK Today!
Visual Guide
References
- Combining Cocatalyst and Oxygen Vacancy to Synergistically Improve Fe2O3 Photoelectrochemical Water Oxidation Performance. DOI: 10.3390/cryst15010085
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the role of a laboratory high-temperature furnace in LLZO crystal phase regulation? Optimize Li-Ion Electrolytes
- What role does a high-performance muffle furnace play in the synthesis of Co3O4 nanoparticles from precursor gels?
- How does temperature control work in modern muffle furnaces? Achieve Unmatched Precision and Efficiency
- Why is alkali fusion in a muffle furnace required for coal fly ash zeolite synthesis? Unlock Maximum Chemical Potential
- What role does a high-temperature muffle furnace play in the pre-calcination of PZT ceramics? Essential Synthesis Guide
- What types of analyses can be performed using a muffle furnace in coal analysis? Unlock Key Coal Quality Insights
- What is the temperature of a muffle furnace? Find Your Ideal Range (300°C to 1800°C+)
- What are some key features of premium muffle furnaces? Unlock Superior Performance and Safety



















