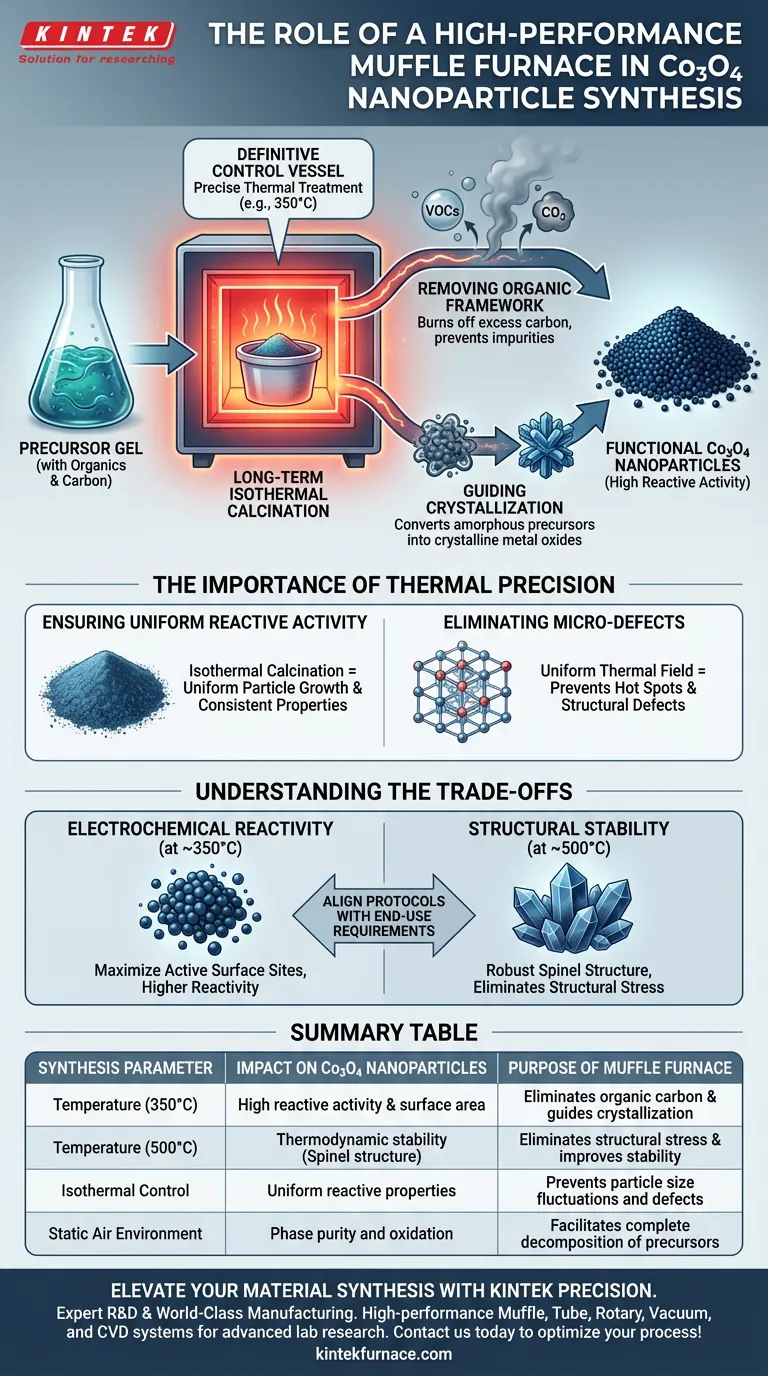
A high-performance muffle furnace acts as the definitive control vessel for converting precursor gels into functional Cobalt Oxide (Co3O4) nanoparticles. Its primary role is to execute long-term isothermal calcination, typically at specific temperatures such as 350°C. This precise thermal treatment is essential for eliminating excess carbon from the precursor material and guiding the crystallization process to ensure the final nanoparticles possess the high reactive activity required for electrochemical applications.
The Core Transformation The muffle furnace does more than simply dry the material; it dictates the final chemical identity of the nanoparticle. By maintaining a uniform thermal field, the furnace ensures the complete decomposition of organic components and the arrangement of cobalt atoms into a highly active crystalline structure.

The Mechanism of Phase Transformation
Removing the Organic Framework
The initial precursor is often a gel containing organic components or carbon. The muffle furnace provides a stable oxidizing environment (static air) required to burn off these elements.
At temperatures around 350°C, the furnace facilitates the thorough removal of excess carbon. This prevents impurities from clogging the surface of the final nanoparticle, which is vital for electrochemical performance.
Guiding Crystallization
Once the organic framework is removed, the remaining cobalt species must form a specific lattice structure. The furnace acts as a guide for this atomic arrangement.
Through controlled heating, the furnace converts amorphous precursor materials into crystalline metal oxides. This step determines the material's phase purity, ensuring the formation of the desired Cobalt Oxide (Co3O4) rather than other transitional phases.
The Importance of Thermal Precision
Ensuring Uniform Reactive Activity
For electrochemical applications, the surface activity of the nanoparticle is paramount. The primary reference highlights that isothermal calcination (holding a constant temperature) is the key to achieving this.
Fluctuations in temperature can lead to uneven particle growth. A high-performance furnace prevents this, ensuring that the resulting powder has consistent reactive properties throughout the batch.
Eliminating Micro-Defects
Advanced insulation and programming in modern furnaces create a uniform thermal field. This prevents "hot spots" or "cold spots" inside the chamber.
Uniformity is critical to preventing micro-defects during the sintering or calcination phase. Such defects could otherwise compromise the structural integrity or conductivity of the final oxide.
Understanding the Trade-offs
Reactivity vs. Stability
While the primary reference emphasizes processing at 350°C for high reactive activity, supplementary data suggests that higher temperatures (e.g., 500°C) are used for thermodynamic stability.
There is a trade-off here: Lower temperatures (300°C–350°C) generally yield higher surface area and reactivity, which is ideal for catalysis or batteries.
Conversely, higher temperatures (500°C) create a more thermodynamically stable spinel structure and eliminate structural stress, but may reduce the active surface area due to sintering.
Process Time vs. Purity
Achieving high purity requires long-term exposure to heat to ensure all volatiles are removed.
Rushing this process with rapid heating can trap carbon inside the particle. You must rely on the furnace's ability to maintain stable temperatures over extended periods to guarantee complete conversion.
Making the Right Choice for Your Goal
To optimize your synthesis of Co3O4 nanoparticles, align your furnace protocols with your specific end-use requirements:
- If your primary focus is Electrochemical Reactivity: Calcine at approximately 350°C to maximize active surface sites while ensuring sufficient carbon removal.
- If your primary focus is Structural Stability: Increase the temperature to 500°C to establish a robust spinel structure and eliminate internal structural stresses, preparing the material for harsh physical processing.
- If your primary focus is High Purity: Utilize a furnace with strict isothermal control to ensure the complete decomposition of citrates and volatile impurities before the final crystallization phase.
The muffle furnace is not just a heater, but a precision instrument that defines the balance between particle purity and reactive performance.
Summary Table:
| Synthesis Parameter | Impact on Co3O4 Nanoparticles | Purpose of Muffle Furnace |
|---|---|---|
| Temperature (350°C) | High reactive activity & surface area | Eliminates organic carbon & guides crystallization |
| Temperature (500°C) | Thermodynamic stability (Spinel structure) | Eliminates structural stress & improves stability |
| Isothermal Control | Uniform reactive properties | Prevents particle size fluctuations and defects |
| Static Air Environment | Phase purity and oxidation | Facilitates complete decomposition of precursors |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect balance between reactivity and structural stability in Co3O4 nanoparticles requires uncompromising thermal control. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of advanced lab research.
Whether you need precise isothermal calcination or a fully customizable high-temperature solution for unique nanomaterial synthesis, our engineering team is ready to help you optimize your process. Contact us today to find the ideal furnace for your laboratory!
Visual Guide
References
- Changwei Shan, Liwei Mi. Co<sub>1−<i>x</i></sub>S@CNT composite with a three-dimensional skeleton for high-performance magnesium–lithium hybrid batteries. DOI: 10.1039/d3ma01089a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How does a laboratory muffle furnace facilitate the biomass carbonization process? Achieve Precise Biochar Production
- What is the function of the temperature control system in a box furnace? Achieve Precise Thermal Processing
- What is the difference between a muffle furnace and an external gas-fired fluidized furnace? Choose the Right High-Temperature Furnace for Your Lab
- What role does a muffle furnace play in SCS of catalysts? Optimize Thermal Initiation for Manganese-Nickel Synthesis
- How is an industrial muffle furnace used to assess the ash content of biomass fibers? Master High-Temp Calcination
- Why use an explosion-proof oven for silica aerogels? Essential Safety for High-Temp Ambient Pressure Drying
- How does the two-stage heating program of a muffle furnace influence the quality of rice husk ash? Optimize Your Silica
- How does a lab box furnace with PID control aid aluminum-doped graphitic carbon synthesis? Precision Thermal Stability



















