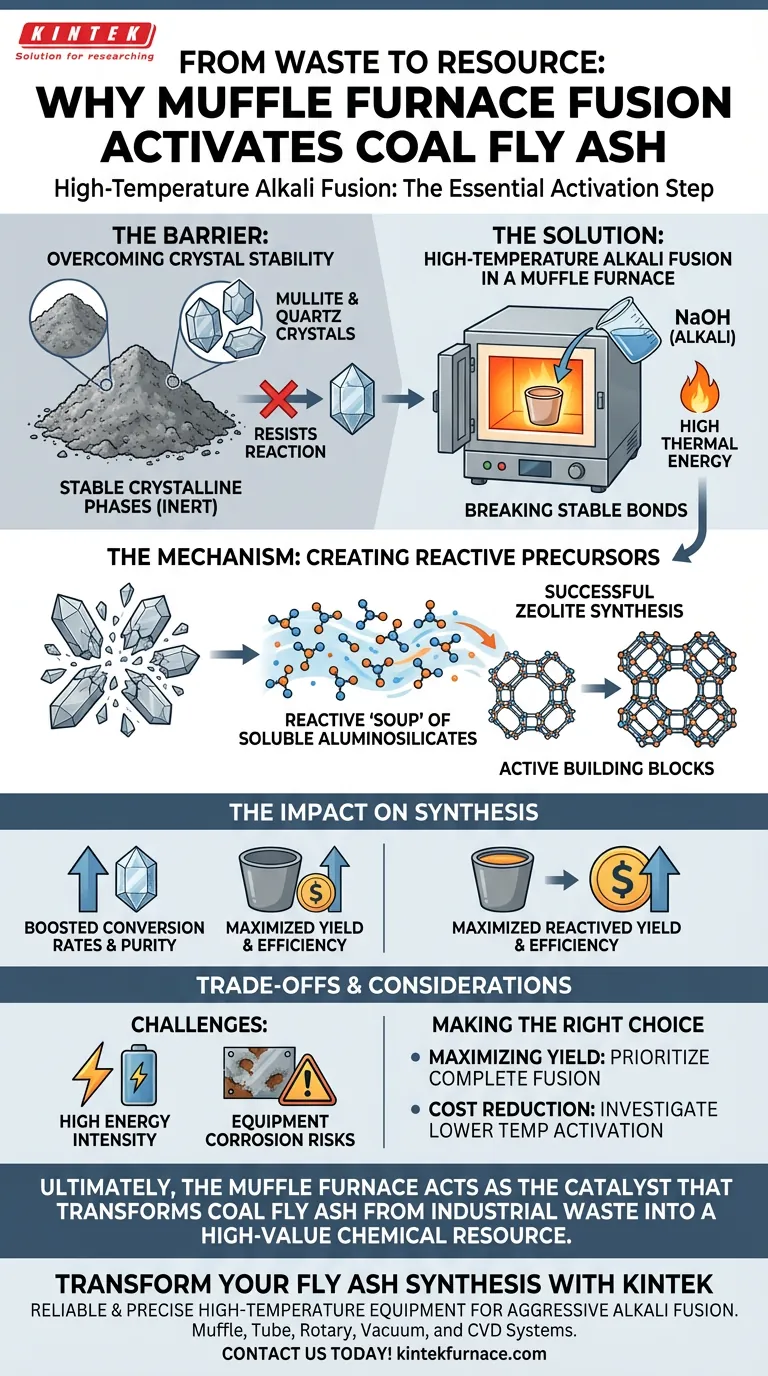
High-temperature alkali fusion is the essential "activation" step required to unlock the chemical potential of coal fly ash. By subjecting the ash to high thermal energy in a muffle furnace alongside sodium hydroxide, you physically break down the chemically stable crystalline phases—specifically mullite and quartz—that otherwise resist reaction. This transformation converts inert minerals into active, soluble aluminosilicate salts, creating the necessary feedstock for successful zeolite synthesis.
The Core Takeaway Coal fly ash naturally consists of rigid, unreactive crystal structures that cannot easily form zeolites. Alkali fusion in a muffle furnace provides the intense thermal energy required to shatter these structures, converting them into a reactive "soup" of aluminosilicates that significantly improves the conversion rate in subsequent synthesis stages.

The Barrier: Overcoming Crystal Stability
The Inert Nature of Fly Ash
Coal fly ash is composed largely of mullite and quartz. These are stable crystalline phases with strong chemical bonds that resist breakdown under standard conditions. Without aggressive intervention, they remain chemically inert.
The Need for Thermal Force
Simple mixing or low-temperature treatments are insufficient to disrupt these bonds. The muffle furnace provides a consistent, high-energy environment necessary to overcome the activation energy barrier of these stable crystals.
The Mechanism: How Fusion Works
Breaking Chemical Bonds
When mixed with sodium hydroxide (NaOH) and heated in the muffle furnace (often around 200 °C or higher depending on the specific protocol), a fusion reaction occurs. The thermal energy literally breaks the chemical bonds holding the mullite and quartz lattices together.
Creating Soluble Precursors
As the bonds break, the silicon and aluminum species are released from their rigid structures. They react with the alkali to form soluble aluminosilicate salts. These salts are the active building blocks required to grow the zeolite crystal framework.
The Impact on Synthesis
Boosting Conversion Rates
The primary goal of this pretreatment is efficiency. By converting solid, insoluble minerals into soluble salts before the hydrothermal stage, you ensure that the maximum amount of material is available for reaction.
Ensuring Purity and Yield
This process significantly improves the conversion rate. Without this step, a large portion of the fly ash would remain as unreacted waste, leading to lower yields and lower purity in the final zeolite product.
Understanding the Trade-offs
Energy Intensity
While effective, alkali fusion is an energy-intensive process. Maintaining the high temperatures required for bond breaking in a muffle furnace increases the overall operational cost and carbon footprint of the synthesis process.
Equipment Corrosion Risks
The combination of high heat and strong alkalis (like sodium hydroxide) is highly corrosive. This environment can degrade crucibles and furnace linings over time, requiring careful material selection and maintenance protocols.
Making the Right Choice for Your Goal
To determine how aggressively to apply this treatment, consider your specific project requirements:
- If your primary focus is Maximizing Yield: Prioritize a complete fusion step to fully convert all quartz and mullite into active aluminosilicates, ensuring the highest possible conversion rate.
- If your primary focus is Cost Reduction: Investigate if lower temperature activation (sub-fusion) is sufficient for your specific target zeolite, acknowledging that unreacted ash may remain in the final product.
Ultimately, the muffle furnace acts as the catalyst that transforms coal fly ash from industrial waste into a high-value chemical resource.
Summary Table:
| Feature | Description |
|---|---|
| Core Objective | Break stable crystalline bonds in mullite and quartz |
| Key Mechanism | High-energy thermal fusion with NaOH to form soluble aluminosilicates |
| Equipment Used | High-temperature muffle furnace |
| Primary Benefit | Significant increase in zeolite conversion rates and purity |
| Key Challenges | High energy consumption and potential equipment corrosion |
Transform Your Fly Ash Synthesis with KINTEK
To achieve the intense thermal energy required for breaking down stable mullite and quartz, you need reliable and precise high-temperature equipment. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique zeolite synthesis needs.
Whether you are aiming to maximize yield or optimize energy efficiency, our lab furnaces provide the uniform heating and durability essential for aggressive alkali fusion treatments. Contact us today to find the perfect furnace solution for your laboratory!
Visual Guide
References
- Aryandson da Silva, Sibele B. C. Pergher. Synthesis and Cation Exchange of LTA Zeolites Synthesized from Different Silicon Sources Applied in CO2 Adsorption. DOI: 10.3390/coatings14060680
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How is a muffle furnace utilized for AlN crystal post-processing? Optimize Surface Purity via Staged Oxidation
- What was the original purpose of a muffle furnace? Discover Its Evolution for Pure Heat
- What are the key technological advancements in modern muffle furnaces? Boost Precision and Efficiency in Your Lab
- What materials are used in the construction of the muffle furnace? Discover the Key Components for High-Temp Performance
- How is a muffle furnace utilized in the RTAC strategy? Precision Atomic Engineering for High-Performance Catalysts
- What role does a muffle furnace play in the calcination of natural zeolite? Optimize Your Adsorption Studies Today
- What special features make muffle furnaces suitable for certain applications? Discover High-Temperature Precision Solutions
- What role does a laboratory muffle furnace play in the determination of phosphorus content? Essential Dry Ashing Guide



















