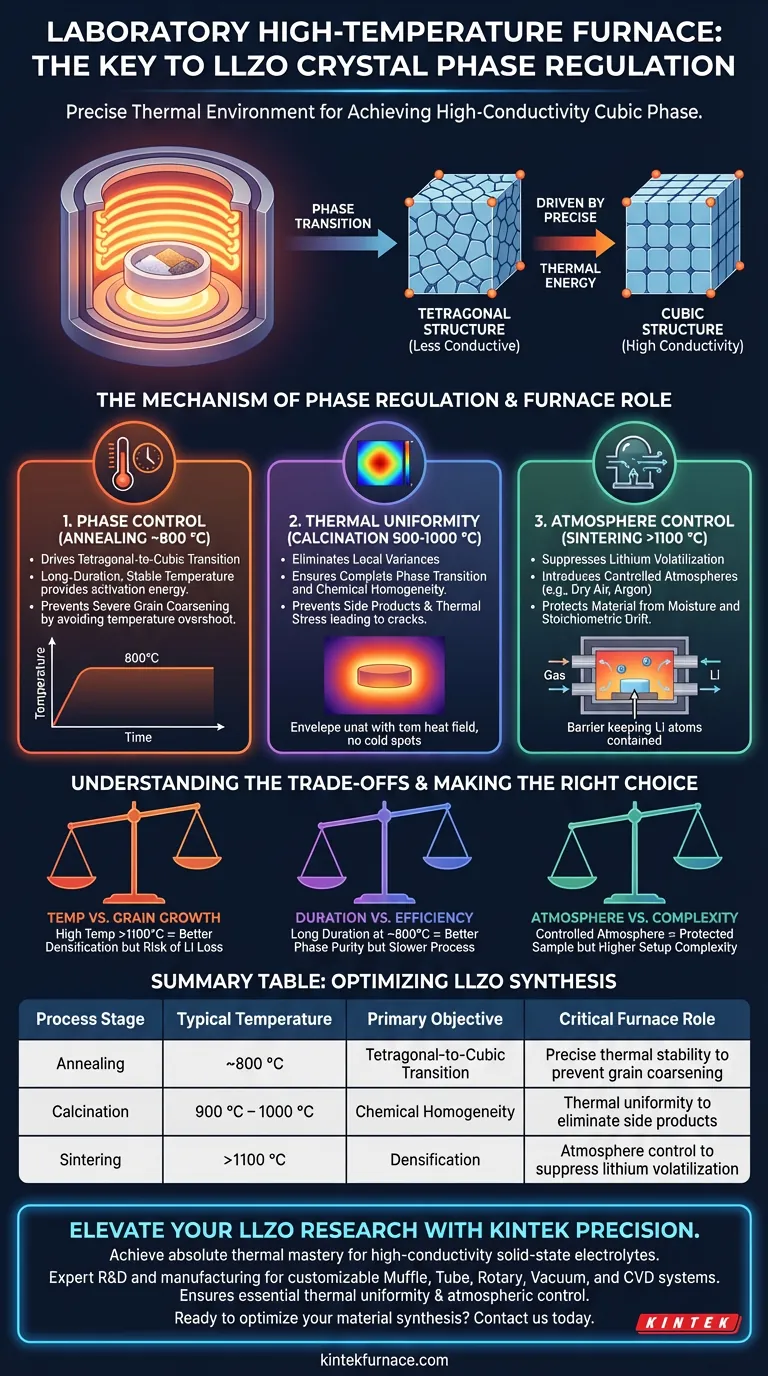
The critical role of a laboratory high-temperature furnace is to provide the precise thermal environment required to drive the phase transition of Li7La3Zr2O12 (LLZO) from a tetragonal to a cubic structure.
This equipment ensures a stable thermal field that facilitates uniform heat transfer. By maintaining exact temperatures (typically around 800 °C for annealing) over long durations, the furnace allows the material to achieve the desired crystal phase without suffering from severe grain coarsening or performance degradation associated with excessive heat.
Core Takeaway Achieving the high-conductivity cubic phase in LLZO is not just about reaching a specific temperature; it requires a controlled "thermal history." The furnace acts as the regulatory mechanism that balances the energy needed for phase transformation against the risks of lithium loss and microstructural degradation.

The Mechanism of Phase Regulation
Driving the Tetragonal-to-Cubic Transition
The primary function of the furnace during the annealing stage is phase control. The user must navigate a specific thermal window—often around 800 °C—to convert the material from a tetragonal structure to the preferred cubic structure.
The furnace maintains this temperature for an extended duration. This long-duration annealing provides the necessary activation energy for the structural rearrangement while keeping the temperature low enough to preserve the material's integrity.
Preventing Microstructural Degradation
Precise temperature regulation is vital to avoid "overshoot." If the temperature spikes uncontrolled, it can lead to severe grain coarsening.
Large, coarse grains can degrade the electrochemical performance of the final electrolyte. The furnace’s ability to hold a steady temperature ensures the phase change occurs without ruining the microstructure.
Thermal Uniformity and Atmosphere Control
Eliminating Local Variances
In processes like calcination (900°C–1000°C) and sintering (>1100°C), thermal uniformity is non-negotiable. Muffle and box furnaces are designed to envelop the sample in a consistent heat field.
If the heat is uneven, the material may suffer from incomplete phase transitions or the formation of side products in cooler zones. Uniformity also prevents thermal stress, which causes cracks to form within the ceramic pellets during densification.
Controlling Lithium Volatilization
Lithium is volatile at high temperatures. High-temperature tube furnaces and vacuum furnaces address this by allowing the introduction of specific atmospheres, such as dry air or argon.
This controlled environment suppresses lithium volatilization and protects the material from environmental moisture. Maintaining the correct stoichiometry is essential, as lithium loss will revert the material structure or reduce ionic conductivity.
Understanding the Trade-offs
High Temperature vs. Grain Growth
Higher temperatures (above 1100°C) are often necessary for densification and grain growth, which improves ionic conductivity. However, pushing the temperature too high risks excessive lithium loss and structural instability.
Annealing Duration vs. Efficiency
Long-duration annealing at lower temperatures (around 800°C) is safer for phase purity and prevents coarsening. The trade-off is process time. You are sacrificing speed to ensure the transition to the cubic phase is complete and the microstructure remains fine.
Atmosphere vs. Complexity
Using vacuum or inert gas environments protects the sample but adds complexity to the setup. Neglecting this variable can lead to moisture contamination (from hygroscopic precursors like LiOH) or stoichiometric drift, rendering the precise thermal profile useless.
Making the Right Choice for Your Goal
To optimize the synthesis of LLZO, align your furnace usage with your specific processing stage:
- If your primary focus is Phase Purity (Cubic Structure): Prioritize stable, long-duration annealing around 800°C to ensure the tetragonal-to-cubic transition without grain coarsening.
- If your primary focus is Densification: Utilize temperatures above 1100°C in a controlled atmosphere (Tube/Vacuum furnace) to promote sintering while suppressing lithium volatilization.
- If your primary focus is Chemical Consistency: Ensure excellent thermal uniformity during calcination (900–1000°C) to prevent side products and local phase segregation.
The furnace is not merely a heat source; it is the precision instrument that dictates whether your LLZO achieves high ionic conductivity or fails due to structural impurities.
Summary Table:
| Process Stage | Typical Temperature | Primary Objective | Critical Furnace Role |
|---|---|---|---|
| Annealing | ~800 °C | Tetragonal-to-Cubic Transition | Precise thermal stability to prevent grain coarsening |
| Calcination | 900 °C – 1000 °C | Chemical Homogeneity | Thermal uniformity to eliminate side products |
| Sintering | >1100 °C | Densification | Atmosphere control to suppress lithium volatilization |
Elevate Your LLZO Research with KINTEK Precision
Achieving the high-conductivity cubic phase in solid-state electrolytes requires more than just heat; it requires absolute thermal mastery. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your unique research needs.
Whether you are scaling up sintering or perfecting annealing profiles, our equipment ensures the thermal uniformity and atmospheric control essential for preventing lithium loss and structural instability.
Ready to optimize your material synthesis? Contact us today to consult with our experts on the ideal high-temperature solution for your laboratory.
Visual Guide
References
- T. Y. Park, Dong‐Min Kim. Low-Temperature Manufacture of Cubic-Phase Li7La3Zr2O12 Electrolyte for All-Solid-State Batteries by Bed Powder. DOI: 10.3390/cryst14030271
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What types of facilities typically use box furnaces? Essential for Labs and Small-Scale Production
- What is the purpose of the port at the rear of the chamber in a muffle furnace? Unlock Precision Control for Your Lab
- Why use a high-temp box resistance furnace for Ca2.5Ag0.3Sm0.2Co4O9 sintering? Ensure Phase Purity and Alignment
- How does a high-precision programmable muffle furnace improve creep testing? Ensure Accuracy in Titanium Alloys
- How should samples be handled when burning or melting them in a muffle furnace? Ensure Safe and Accurate Results
- Why is it important to check the power supply of a muffle furnace? Ensure Safety and Accurate Results
- How do box type high-temperature resistance furnaces address environmental and energy concerns? Boost Efficiency and Sustainability
- What are the common uses of muffle furnaces in laboratory settings? Essential for Ashing, Heat Treatment, and Sintering



















