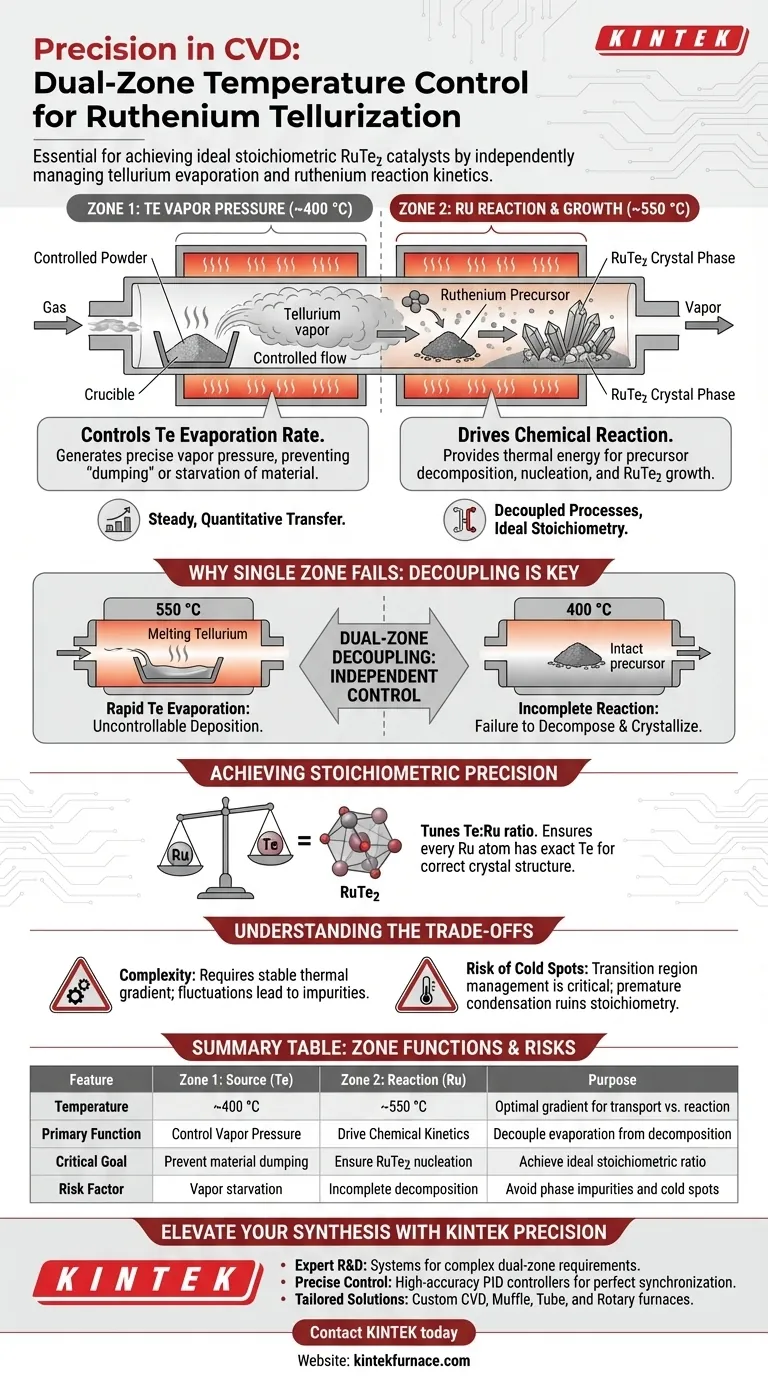
Precise dual-zone temperature control is required to independently manage the evaporation rate of the tellurium source and the chemical reaction kinetics of the ruthenium precursor. By maintaining the tellurium powder at approximately 400 °C and the reaction zone at 550 °C, the system ensures that the supply of tellurium vapor perfectly matches the decomposition rate needed to form the catalyst.
By decoupling the source temperature from the reaction temperature, you ensure a steady, quantitative transfer of material, which is the only way to achieve the ideal stoichiometric ratio for the RuTe2 crystalline phase.

The Mechanics of Dual-Zone Tellurization
Zone 1: Controlling Vapor Pressure
The first zone, set to approximately 400 °C, focuses exclusively on the tellurium powder.
At this specific temperature, the system generates the precise vapor pressure required to transport the tellurium downstream.
This prevents the "dumping" of excess material that would occur at higher temperatures or the starvation of the reaction that would occur at lower temperatures.
Zone 2: Driving the Reaction
The second zone, maintained at 550 °C, is where the actual synthesis occurs.
This higher thermal energy is necessary to decompose the ruthenium precursor effectively.
It also provides the thermodynamic conditions required for the nucleation and growth of the RuTe2 crystalline phase.
Why a Single Temperature Fails
Decoupling Physical and Chemical Processes
In a single-zone system, you are forced to compromise between evaporation and reaction.
If you heat the entire system to 550 °C (the reaction temperature), the tellurium would evaporate too rapidly, leading to uncontrollable deposition rates.
Conversely, if you held the system at 400 °C (the evaporation temperature), the ruthenium precursor would likely fail to decompose or crystallize properly.
Ensuring Stoichiometric Precision
The primary goal of this process is to form RuTe2 with an ideal stoichiometric ratio.
Dual-zone control allows you to "tune" the ratio of tellurium vapor to ruthenium availability.
This balance ensures that every ruthenium atom has access to the exact amount of tellurium needed to form the correct crystal structure.
Understanding the Trade-offs
Complexity vs. Control
While a dual-zone setup offers superior control, it introduces complexity in calibration.
You must ensure that the thermal gradient between the 400 °C zone and the 550 °C zone is stable; fluctuations in the gradient can lead to phase impurities.
The Risk of Cold Spots
Maintaining two distinct zones requires careful management of the transition region between them.
If the temperature dips below 400 °C in the transport path between zones, tellurium vapor may condense prematurely before reaching the ruthenium.
This results in a non-quantitative transfer, ruining the stoichiometry of the final catalyst.
Making the Right Choice for Your Goal
To achieve the best results in ruthenium tellurization, consider your specific priorities:
- If your primary focus is Phase Purity: Maintain the reaction zone strictly at 550 °C to ensure the RuTe2 crystalline phase forms without secondary byproducts.
- If your primary focus is Stoichiometry: Prioritize the stability of the 400 °C source zone to guarantee a steady, quantitative stream of tellurium vapor.
Success in this process relies not just on reaching these temperatures, but on maintaining the distinct separation between them.
Summary Table:
| Feature | Zone 1: Source (Te) | Zone 2: Reaction (Ru) | Purpose |
|---|---|---|---|
| Temperature | ~400 °C | ~550 °C | Optimal gradient for transport vs. reaction |
| Primary Function | Control Vapor Pressure | Drive Chemical Kinetics | Decouple evaporation from decomposition |
| Critical Goal | Prevent material dumping | Ensure RuTe2 nucleation | Achieve ideal stoichiometric ratio |
| Risk Factor | Vapor starvation | Incomplete decomposition | Avoid phase impurities and cold spots |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect stoichiometric ratio in RuTe2 catalysts requires more than just heat; it requires absolute thermal decoupling. KINTEK provides advanced, customizable CVD systems, Muffle, Tube, and Rotary furnaces designed to eliminate cold spots and maintain stable thermal gradients.
Why choose KINTEK?
- Expert R&D: Our systems are engineered for complex dual-zone and multi-zone requirements.
- Precise Control: High-accuracy PID controllers ensure your 400°C source and 550°C reaction zones remain perfectly synchronized.
- Tailored Solutions: From vacuum integration to unique tube configurations, we build the tools your research demands.
Ready to optimize your chemical vapor deposition process? Contact KINTEK today to discuss your custom furnace needs with our engineering team.
Visual Guide
References
- Mehtap Aygün. RuTe2 Decorated Carbon Nanofiber Electrocatalyst Synthesized via a Sustainable Method for Electrochemical Hydrogen Evolution in Acidic and Alkaline Electrolytes. DOI: 10.21597/jist.1647816
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is it necessary to adjust sample position in deposition chambers for large-scale tantalum-carbon composites?
- What is a CVD machine? Build High-Performance Materials from Gas with Precision
- What is an example of chemical vapor deposition? Building the Microchips in Your Electronics
- What is the configuration of CVD furnaces? Unlock Precision Thin Film Deposition
- What is the basic configuration of CVD coating equipment? Unlock High-Quality Thin Film Deposition
- What temperature ranges can a CVD Tube Furnace achieve with different tube materials? Unlock High-Temp Precision for Your Lab
- What are the key differences between PVD and CVD in terms of deposition mechanism? Choose the Right Coating Method for Your Lab
- What are some biomedical applications of CVD? Enhance Medical Device Safety and Longevity



















