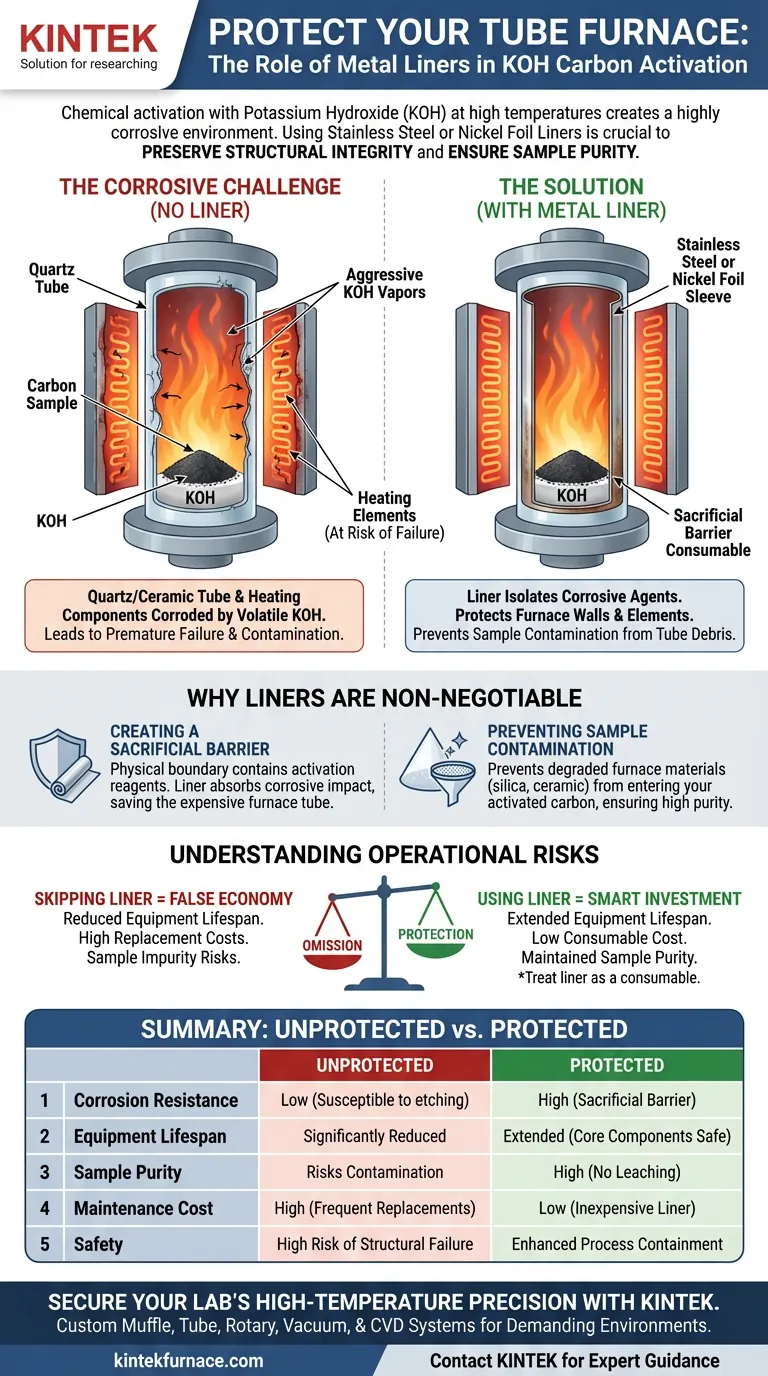
Preserving the structural integrity of your tube furnace is the primary reason for using protective liners during chemical activation. When potassium hydroxide (KOH) is heated to activation temperatures, it becomes a highly aggressive corrosive agent that attacks standard quartz or ceramic furnace tubes. Placing a sleeve of stainless steel or nickel foil inside the tube acts as a critical barrier, preventing the reagent from destroying the furnace walls and protecting the heating elements from failure.
The activation process turns potassium hydroxide into a powerful etching agent that does not distinguish between your carbon sample and the furnace tube. A metal foil liner effectively isolates this corrosive environment, extending the lifespan of your expensive equipment and maintaining the chemical purity of your materials.

The Corrosive Challenge of KOH Activation
The Mechanism of Damage
While the goal of using potassium hydroxide (KOH) is to etch the carbon skeleton and create a porous structure, its chemical aggression is not limited to the sample.
At high temperatures, KOH becomes volatile and highly reactive. It actively corrodes the quartz or ceramic materials typically used to construct the inner walls of tube furnaces.
Threat to Heating Components
Without protection, the corrosive reaction extends beyond the tube walls.
Once the inner vessel is compromised, the corrosive vapors can reach and degrade the furnace's core heating components. This leads to premature equipment failure and costly repairs.
Why Metal Liners are Non-Negotiable
Creating a Sacrificial Barrier
Stainless steel or nickel foil liners function as an isolation sleeve.
By placing these metals inside the furnace tube, you create a physical boundary that contains the activation reagents. The liner absorbs the corrosive impact, sacrificing itself to keep the structural furnace tube intact.
Preventing Sample Contamination
Protecting the furnace is not just about equipment longevity; it is also about sample quality.
When KOH corrodes a quartz or ceramic tube, the structural material degrades and creates impurities. Using a liner prevents these foreign substances from entering the reaction vessel, ensuring your activated carbon is not contaminated by debris from the furnace walls.
Understanding the Operational Risks
The Consequence of Omission
Skipping the use of a liner is a false economy.
While it may save setup time, the direct exposure of the furnace tube to KOH guarantees a significantly reduced equipment lifespan. The cost of replacing a quartz tube or heating element far outweighs the cost of a foil liner.
Limitations of the Liner
It is important to recognize that the liner is not permanent.
Because it takes the brunt of the corrosive attack, the foil itself will eventually degrade. It should be treated as a consumable consumable and inspected or replaced regularly to maintain effective isolation.
Ensuring Process Integrity
To maximize both your equipment ROI and the quality of your carbon materials, consider the following:
- If your primary focus is Equipment Longevity: Always verify that the foil liner creates a complete barrier between the reagents and the quartz tube to prevent irreversible etching.
- If your primary focus is Material Purity: Inspect liners frequently for breaches to ensure that dissolved furnace materials are not being introduced into your high-performance supercapacitor samples.
By isolating the corrosive power of KOH, you ensure that the etching process remains focused on the carbon material, not your laboratory hardware.
Summary Table:
| Feature | Quartz/Ceramic Tube (Unprotected) | With Stainless/Nickel Foil Liner |
|---|---|---|
| Corrosion Resistance | Low (Susceptible to KOH etching) | High (Acts as a sacrificial barrier) |
| Equipment Lifespan | Significantly reduced due to damage | Extended by protecting core components |
| Sample Purity | Risks contamination from tube debris | High (Prevents leaching of silica/ceramic) |
| Maintenance Cost | High (Frequent tube/element replacement) | Low (Inexpensive consumable foil replacement) |
| Safety | High risk of structural failure | Enhanced process containment |
Secure Your Lab’s High-Temperature Precision with KINTEK
Don't let aggressive chemical activation compromise your research. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to withstand your most demanding chemical environments. Whether you are developing high-performance supercapacitors or advanced porous materials, our engineering team ensures your equipment provides the longevity and purity your work deserves.
Ready to upgrade your thermal processing capabilities? Contact KINTEK today for expert guidance and custom furnace solutions.
Visual Guide
References
- Giovanni Zuccante, Carlo Santoro. Transforming Cigarette Wastes into Oxygen Reduction Reaction Electrocatalyst: Does Each Component Behave Differently? An Experimental Evaluation. DOI: 10.1002/celc.202300725
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What are tube furnaces made of? Choose the Right Material for Your Thermal Process
- What is a multi zone tube furnace used for? Unlock Precision Thermal Processing for Advanced Materials
- How does a hydrogen reduction environment in an industrial tube furnace facilitate gold-copper alloy microspheres?
- What is the heating rate of a tube furnace? Balancing Speed with Safety for Your Lab
- How does the uniform thermal field provided by a vertical tube resistance furnace impact phase equilibrium experiments?
- What are the typical working temperature ranges for lab tube furnaces? Find the Right Furnace for Your Process
- How is a laboratory tube furnace used in new energy research? Unlock Next-Gen Battery and Fuel Cell Materials
- How is sealing and atmosphere control achieved in a tube furnace? Master Precise Gas Environments for Your Lab



















