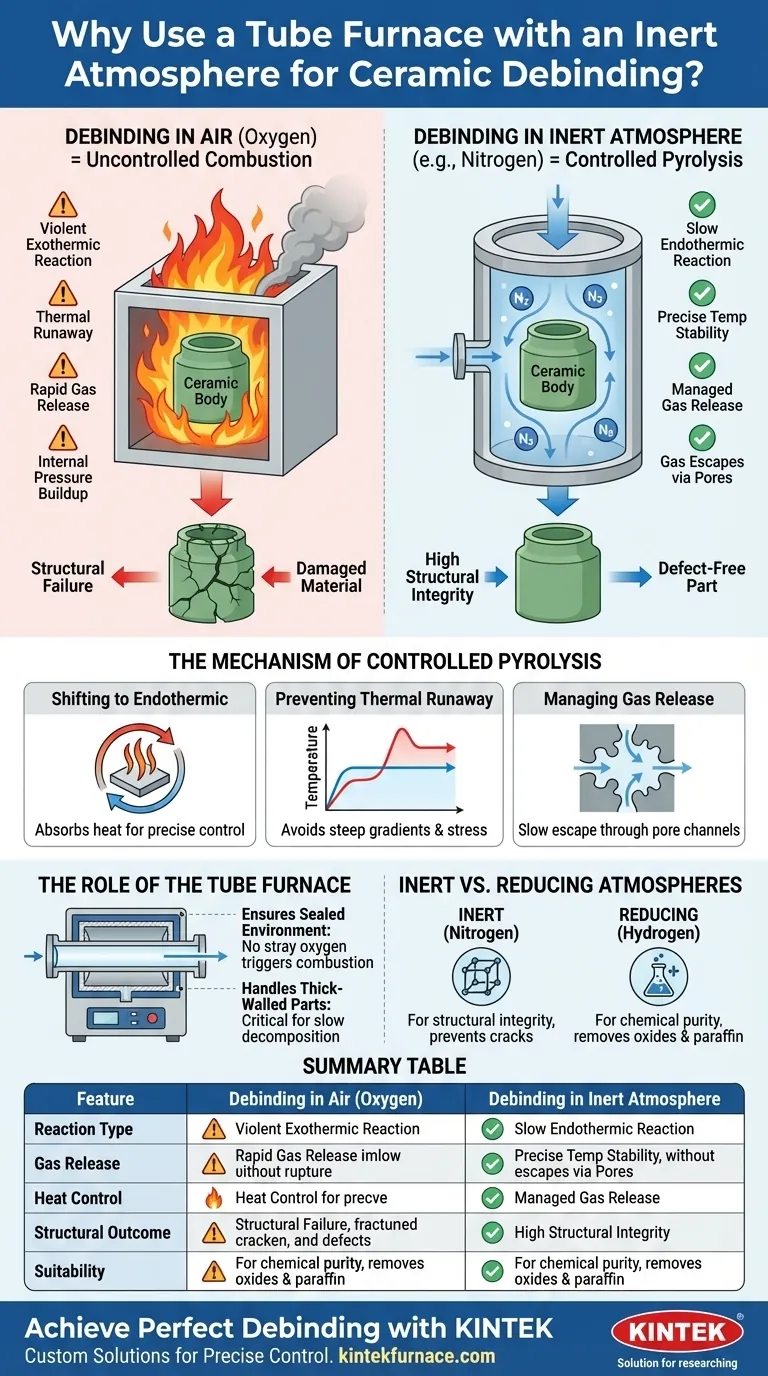
Using a tube furnace with an inert atmosphere is essential to preserve the structural integrity of ceramic green bodies during debinding. By replacing air with a gas like nitrogen, you transform the process from uncontrolled combustion into a slow, endothermic pyrolysis. This prevents violent exothermic reactions that would otherwise cause internal pressure buildup, leading to cracks or explosive failure in the part.
Core Takeaway Processing ceramic green bodies in air causes resin binders to burn violently, generating heat and pressure that damage the material. An inert atmosphere forces the binders to decompose thermally (pyrolysis) rather than burn, ensuring a smooth, controlled release of gases and preventing structural defects.

The Mechanism of Controlled Pyrolysis
Shifting from Exothermic to Endothermic
In an oxygen-rich environment (air), resin binders ignite. This is an exothermic reaction, meaning it generates significant heat rapidly.
In a tube furnace with an inert atmosphere, oxygen is excluded. Consequently, the binders undergo pyrolysis—a thermal decomposition process. This is endothermic, meaning it absorbs heat rather than generating it, allowing for precise temperature control.
preventing Thermal Runaway
When binders burn in air, the internal temperature of the ceramic part can spike uncontrollably.
This "thermal runaway" creates steep temperature gradients within the part. These gradients cause differential expansion, which stresses the ceramic structure and often leads to catastrophic cracking.
Managing Gas Release
Debinding involves turning solid binders into gas. If this happens too fast (as in combustion), the gas expands explosively inside the part.
Controlled pyrolysis in an inert atmosphere generates gas at a manageable rate. This allows the gases to navigate through the pore channels of the green body and escape without rupturing the material.
The Role of the Tube Furnace
Ensuring a Sealed Environment
A tube furnace is specifically designed to maintain a strictly controlled atmosphere.
Unlike standard box furnaces which may leak or fluctuate, a tube furnace ensures the inert gas completely surrounds the part. This guarantees that no stray oxygen triggers localized combustion.
Handling Large or Thick-Walled Parts
Thick ceramic parts are most susceptible to cracking because gases have a longer path to escape.
The tube furnace's ability to maintain a stable, inert environment is critical for these complex geometries. It ensures the slow, steady decomposition required to debind thick walls without failure.
Understanding the Trade-offs: Inert vs. Reducing Atmospheres
While an inert atmosphere (like nitrogen) is excellent for structural integrity during resin removal, it may not suffice for all material compositions. You must evaluate if your material has chemical sensitivities that require a reducing atmosphere (like hydrogen).
When Inert is Insufficient
Inert gases prevent combustion, but they do not actively remove oxides.
If your "green body" contains metals (such as iron or specific alloys) alongside the ceramic, trace oxygen or moisture can still cause oxidation at high temperatures.
The Role of Hydrogen (Reducing Atmosphere)
As noted in supplementary contexts, a hydrogen atmosphere plays a different role. It actively strips oxygen from the environment and the material.
This is necessary when you need to remove paraffin binders thoroughly or when you must prevent the oxidation of metallic elements to ensure high chemical purity before sintering.
Making the Right Choice for Your Goal
Select your furnace atmosphere based on the specific risks associated with your binder type and material composition.
- If your primary focus is preventing cracks in ceramics: Use an inert atmosphere (Nitrogen) to force endothermic pyrolysis and avoid violent combustion of resin binders.
- If your primary focus is chemical purity in metal-containing parts: Use a reducing atmosphere (Hydrogen) to prevent oxidation of alloys and facilitate the removal of paraffin binders.
- If your primary focus is processing thick-walled components: Use a tube furnace to guarantee the consistent, sealed environment required for slow gas release.
By controlling the atmosphere, you convert a chaotic burning process into a precision engineering step, ensuring your parts emerge defect-free.
Summary Table:
| Feature | Debinding in Air (Oxygen) | Debinding in Inert Atmosphere |
|---|---|---|
| Reaction Type | Exothermic (Combustion) | Endothermic (Pyrolysis) |
| Gas Release | Rapid/Violent (Explosive) | Slow & Controlled (Steady) |
| Heat Control | High risk of thermal runaway | Precise temperature stability |
| Structural Outcome | Prone to cracks and defects | High structural integrity |
| Suitability | Simple, thin-walled parts | Complex or thick-walled ceramics |
Achieve Perfect Debinding with KINTEK
Don't let uncontrolled combustion compromise your ceramic components. KINTEK offers expertly engineered Muffle, Tube, Rotary, Vacuum, and CVD systems designed to provide the precise atmosphere control your materials demand.
Backed by industry-leading R&D and manufacturing, our high-temperature lab furnaces are fully customizable to handle nitrogen, hydrogen, or vacuum environments. Whether you are processing thick-walled ceramics or delicate alloys, KINTEK ensures your parts emerge defect-free and chemically pure.
Ready to optimize your thermal processing? Contact us today to find your custom solution!
Visual Guide
References
- Yun-Zhuo Zhang, Yousheng Zou. Pyrolysis Kinetics-Driven Resin Optimization for Enhanced Reliability in Ceramic Vat Photopolymerization Manufacturing. DOI: 10.3390/ma18174004
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the key advantages of using fluidized bed technology in vertical tube furnaces? Boost Efficiency and Uniformity
- What environmental conditions does a tube furnace provide for CFeS aerogels? Master Carbonization & Inert Protection
- What is the function of a dual-temperature zone tube furnace in CVD? Enhance MoS2/GaN Synthesis Precision
- What is the function of a Tube Furnace in the thermal oxidation of Ti6Al4V alloy? Enhance Hardness & Wear Resistance
- What role does a tube furnace play in tantalum capacitor recycling? Enhancing Metal Recovery Through Pyrolysis
- What role does a tubular furnace play in converting precursors into microwave-absorbing Fe-CN@CoCN? Expert Insights
- How does air annealing in a tube furnace enhance the performance of TiO2 nanorods? Boost Crystallinity and Conductivity
- How are tubular furnaces used in chemical synthesis? Unlock Precise Material Creation



















