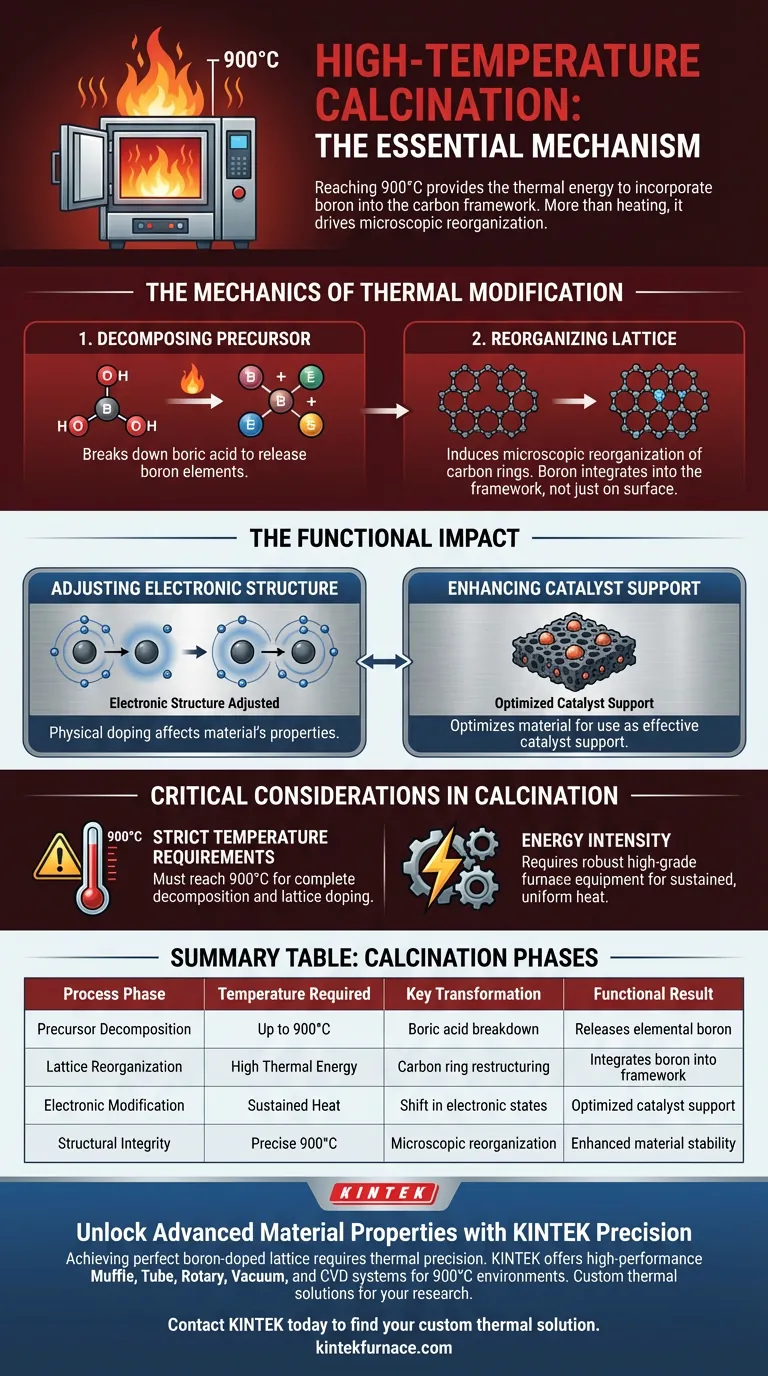
High-temperature calcination is the essential mechanism required to successfully incorporate boron atoms into a carbon framework. By subjecting the material to intense heat—specifically reaching up to 900 degrees Celsius—you provide the thermal energy necessary to decompose precursors like boric acid and physically force boron elements into the carbon lattice.
High-temperature calcination does more than simply heat the material; it drives a microscopic reorganization of the carbon rings. This structural shift is the only way to effectively dope boron into the lattice, thereby altering the electronic structure for use as a catalyst support.

The Mechanics of Thermal Modification
Decomposing the Precursor
The process begins with the breakdown of the boron source, typically boric acid.
Standard thermal environments are insufficient for this task; a high-temperature furnace is required to fully decompose the acid and release the boron elements.
Reorganizing the Lattice
Once the boron is released, the extreme heat induces a microscopic reorganization of the porous carbon.
Specifically, the six-membered ring structures of the carbon begin to shift and restructure.
This reorganization creates the necessary conditions for boron atoms to integrate directly into the carbon framework rather than merely resting on the surface.
The Functional Impact
Adjusting Electronic Structure
The physical doping of boron atoms has a profound effect on the material's properties.
It effectively adjusts the electronic structure of the porous carbon.
Enhancing Catalyst Support
This electronic modification is the primary reason for the process.
It optimizes the material to serve as a highly effective catalyst support, a capability that untreated porous carbon lacks.
Critical Considerations in Calcination
Strict Temperature Requirements
Precision is paramount; the process demands temperatures reaching 900 degrees Celsius.
Failing to reach this threshold will result in incomplete decomposition of the boric acid and a failure to dope the lattice.
Energy Intensity
This method is inherently energy-intensive due to the extreme thermal requirements.
It necessitates robust, high-grade furnace equipment capable of sustaining these temperatures to ensure the reorganization is uniform.
Optimizing Your Synthesis Strategy
To achieve the best results in modifying boron-doped porous carbon, align your process with your specific technical goals.
- If your primary focus is structural integrity: Ensure your thermal profile reaches the full 900°C to guarantee the complete microscopic reorganization of the carbon rings.
- If your primary focus is catalytic efficiency: Verify that the calcination duration is sufficient to fully adjust the electronic structure of the catalyst support.
Mastering the high-temperature calcination phase is the decisive factor in unlocking the advanced electronic properties of doped carbon materials.
Summary Table:
| Process Phase | Temperature Required | Key Transformation | Functional Result |
|---|---|---|---|
| Precursor Decomposition | Up to 900°C | Boric acid breakdown | Releases elemental boron |
| Lattice Reorganization | High Thermal Energy | Carbon ring restructuring | Integrates boron into framework |
| Electronic Modification | Sustained Heat | Shift in electronic states | Optimized catalyst support |
| Structural Integrity | Precise 900°C | Microscopic reorganization | Enhanced material stability |
Unlock Advanced Material Properties with KINTEK Precision
Achieving the perfect boron-doped lattice requires more than just heat; it requires absolute thermal precision. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to sustain the rigorous 900°C environments needed for your most demanding lab synthesis.
Whether you are modifying porous carbon or developing next-generation catalysts, our customizable high-temperature furnaces provide the uniformity and reliability your research deserves. Contact KINTEK today to find your custom thermal solution and elevate your material science outcomes.
Visual Guide
References
- Hui Liu, Qingshan Zhao. A Palladium Catalyst Supported on Boron-Doped Porous Carbon for Efficient Dehydrogenation of Formic Acid. DOI: 10.3390/nano14060549
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What process conditions does a high-temperature muffle furnace provide for biomass briquette ash analysis?
- What are the primary applications of a high-temperature muffle furnace in biomass fuel evaluation? Optimize Energy Data
- Why is atmosphere control important in a Muffle furnace? Unlock Precise Material Processing
- What temperature range can a box furnace operate in? Find the Perfect Fit for Your Lab's Needs
- What is the function of a forced convection oven during the preparation of TiH2 powder from TiO2? Ensure Purity Now
- What is a Muffle Furnace with Hydrogen atmosphere? Achieve Oxide-Free, Bright Metal Finishes
- Why are muffle furnaces important in laboratories? Essential for Contamination-Free High-Temperature Processing
- What temperature information is displayed simultaneously on the controls? Monitor Real-Time and Target Temperatures for Precision



















