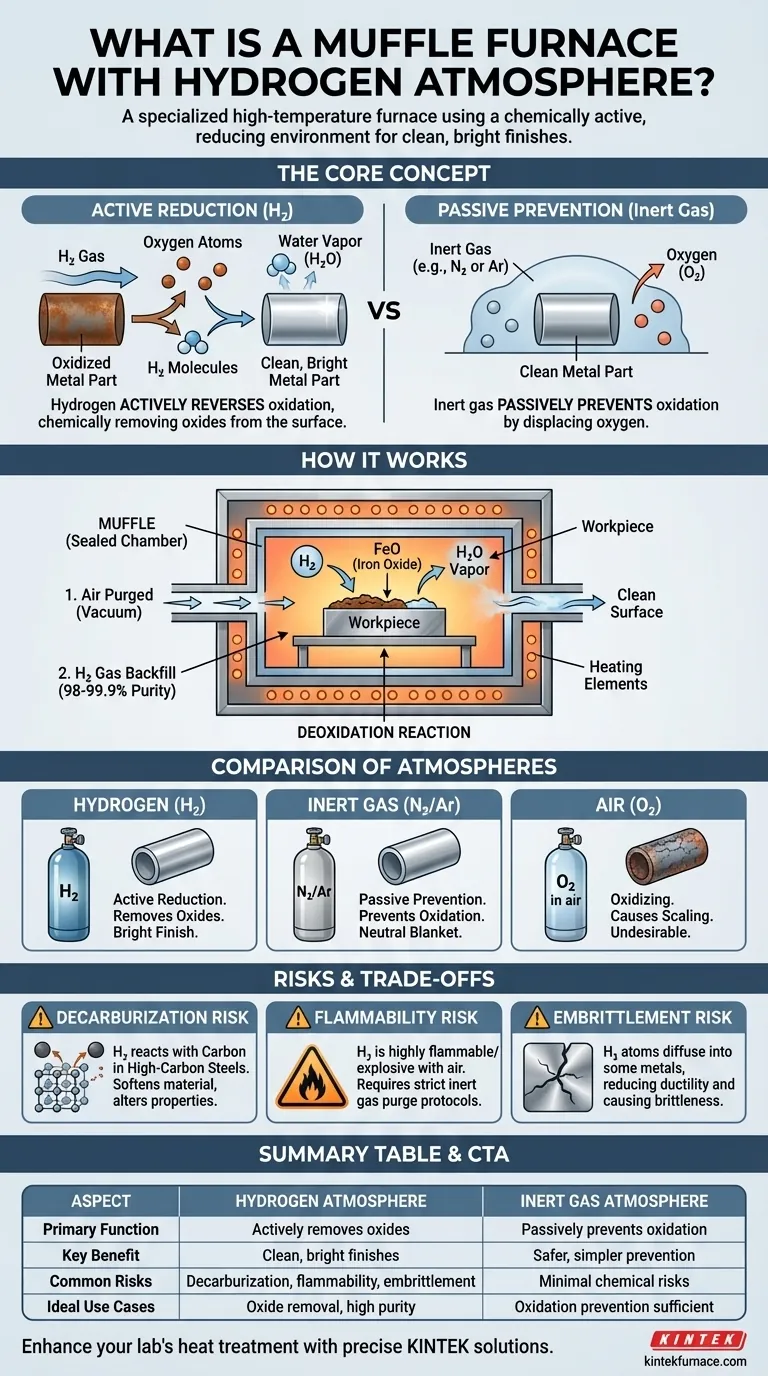
In essence, a muffle furnace with a hydrogen atmosphere is a specialized type of high-temperature furnace that uses a sealed inner chamber, known as a muffle, filled with hydrogen gas. Unlike furnaces that operate in air or inert gas, this setup creates a chemically active, reducing environment. The primary purpose is to remove oxides from the surface of materials during heat treatment, resulting in a clean, bright finish.
The core distinction to understand is that while an inert gas atmosphere (like nitrogen or argon) simply prevents oxidation, a hydrogen atmosphere actively reverses it. It is not a passive blanket but a chemical agent that strips oxygen from the material being processed.
How a Hydrogen Atmosphere Furnace Works
A muffle furnace is designed to create a highly controlled environment, separate from the heating elements and outside air. The introduction of hydrogen transforms it into a powerful tool for metallurgical processing.
The Role of the Muffle
The "muffle" is a sealed tunnel or chamber, typically made of a high-temperature metal alloy. This chamber isolates the parts from the furnace's heating elements.
This design ensures that the carefully controlled atmosphere inside the muffle is not contaminated by the surrounding air or by-products from the heating process.
The Reducing Atmosphere
To create the environment, the muffle is first purged of air. A best practice involves using a vacuum to remove all oxygen and then backfilling with high-purity (98-99.9%) hydrogen gas.
At high temperatures, this hydrogen becomes a potent reducing agent. It aggressively seeks out and reacts with oxygen.
The Deoxidation Reaction
When a metal part has oxides on its surface (a form of rust or tarnish), the hydrogen (H₂) reacts with the metal oxide (e.g., iron oxide, FeO).
The hydrogen effectively "steals" the oxygen atom, forming water vapor (H₂O), which is then safely vented from the furnace. This leaves behind a pure, clean metal surface.
Why Choose Hydrogen Over Other Atmospheres?
The choice of atmosphere is dictated entirely by the desired outcome of the heat treatment process.
Hydrogen vs. Air
This is the most straightforward comparison. Heating metals in air, which contains ~21% oxygen, causes rapid oxidation and scaling. A hydrogen atmosphere does the exact opposite, preventing and removing oxides.
Hydrogen vs. Inert Gas (Nitrogen or Argon)
This is the most critical distinction. Inert gases are non-reactive. They work by creating a neutral blanket that displaces oxygen, thereby passively preventing oxidation from occurring.
Hydrogen, however, is chemically active. It not only prevents oxidation but also actively removes existing oxides from the material's surface. This makes it superior for applications requiring an exceptionally clean, bright finish.
Understanding the Trade-offs and Risks
Using a hydrogen atmosphere offers unique benefits but also introduces significant risks and process limitations that must be carefully managed.
The Risk of Decarburization
At high temperatures, hydrogen can react with the carbon within high-carbon steels. This reaction pulls carbon out of the steel, a process called decarburization.
This loss of carbon can soften the material and fundamentally alter its mechanical properties, which is often highly undesirable for the final product.
The Risk of Flammability
Hydrogen is extremely flammable and can be explosive when mixed with air. Strict safety protocols are non-negotiable.
Furnaces must be purged with an inert gas, like nitrogen, to remove all hydrogen before the doors are opened to the air. This prevents a dangerous reaction between the hot hydrogen and atmospheric oxygen.
Material Compatibility
Not all materials are suitable for processing in hydrogen. Certain metals can suffer from hydrogen embrittlement, where hydrogen atoms diffuse into the metal lattice and reduce its ductility, making it brittle.
Making the Right Choice for Your Process
Selecting the correct furnace atmosphere is critical for achieving the desired metallurgical properties and surface finish.
- If your primary focus is simply preventing oxidation on clean parts: An inert gas atmosphere like nitrogen or argon is often the safer, simpler, and more cost-effective choice.
- If your primary focus is actively removing existing oxides for a bright, clean finish: A hydrogen atmosphere is the superior choice due to its powerful chemical reducing properties.
- If you are processing high-carbon materials: Exercise extreme caution with hydrogen, as the risk of decarburization can compromise the integrity of your material.
Ultimately, matching the chemical properties of the atmosphere to your material and process goals is the key to successful heat treatment.

Summary Table:
| Aspect | Hydrogen Atmosphere | Inert Gas Atmosphere |
|---|---|---|
| Primary Function | Actively removes oxides by chemical reduction | Passively prevents oxidation by displacement |
| Key Benefit | Produces clean, bright metal finishes | Safer and simpler for oxidation prevention |
| Common Risks | Decarburization, flammability, hydrogen embrittlement | Minimal chemical risks, but less effective for oxide removal |
| Ideal Use Cases | Applications requiring oxide removal and high purity | Processes where oxidation prevention is sufficient |
Ready to enhance your lab's heat treatment processes with precise hydrogen atmosphere solutions?
At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnaces tailored for diverse laboratories. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all supported by strong deep customization capabilities to meet your unique experimental needs. Whether you're aiming for oxide-free metal finishes or other specialized applications, our expertise ensures optimal performance and safety.
Contact us today to discuss how our solutions can benefit your specific processes!
Visual Guide
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the significance of temperature control precision in high-temperature furnaces for carbon-doped titanium dioxide?
- Why is a laboratory high-temperature box furnace essential for KNN ceramic powders? Mastering Solid-State Synthesis
- Why is immediate water-quenching required after thermal simulation? Preserve (CoCrNi)94Al3Ti3 Alloy Microstructure
- Why is a box muffle furnace used for the 800°C annealing of titanium LMD samples? Optimize Your Material Performance
- How is a muffle furnace utilized for AlN crystal post-processing? Optimize Surface Purity via Staged Oxidation



















