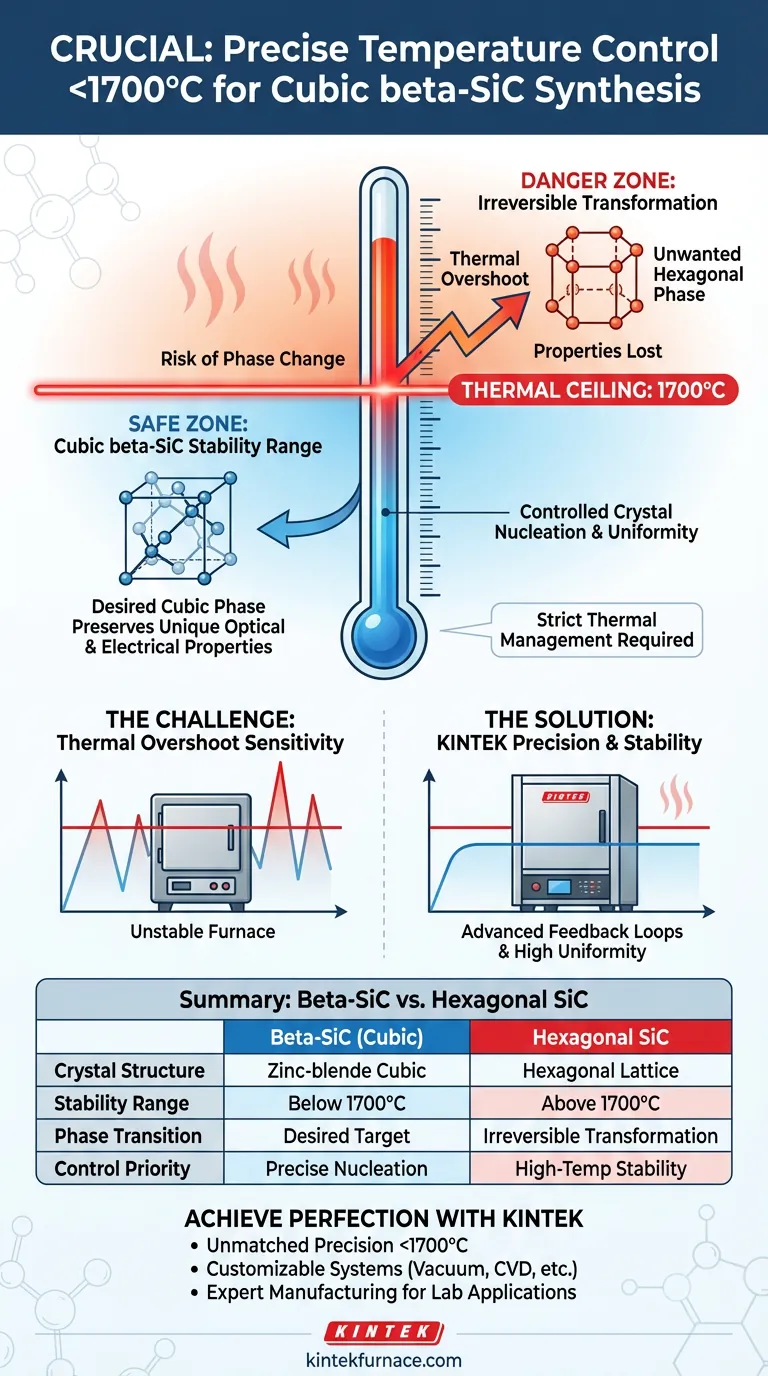
Precise temperature control below 1700°C is critical because cubic beta-Silicon Carbide (beta-SiC) possesses a lower thermodynamic stability range than other SiC variations. Exceeding this temperature threshold triggers an irreversible transformation from the desired cubic zinc-blende structure into a high-temperature stable hexagonal phase. Strict thermal management is required to preserve the specific optical and electrical properties inherent to the cubic phase.
The synthesis of beta-SiC requires a strict thermal ceiling to preserve its unique cubic crystal lattice. By limiting heat to under 1700°C, manufacturers prevent unwanted phase transitions and precisely manage crystal nucleation rates to achieve target material performance.

The Thermodynamics of Silicon Carbide
Stability of the Cubic Phase
Beta-SiC is defined by a cubic zinc-blende structure. This specific crystal arrangement provides unique material characteristics distinct from other forms of silicon carbide.
However, this cubic structure operates within a limited thermodynamic stability range. It is essentially a low-temperature phase that cannot sustain its lattice integrity at extreme heat.
The Risk of Hexagonal Transformation
If the processing temperature exceeds 1700°C, the material undergoes a phase change. The cubic lattice rearranges itself into a hexagonal phase, which is more stable at high temperatures.
Once this transformation occurs, the material is no longer beta-SiC. Consequently, the specific properties sought after in the cubic form are lost.
Controlling Crystal Formation
Managing Nucleation Rates
Temperature control is not just about preventing phase changes; it also governs how the crystals grow. Operating below 1700°C allows for effective control of the crystal nucleation rate.
By regulating this rate, manufacturers can influence the size and quality of the crystals. This precision ensures the material develops a uniform structure necessary for high-performance applications.
Preserving Material Properties
The utility of beta-SiC lies in its specific optical and electrical properties. These properties are a direct result of the cubic zinc-blende structure.
Heating equipment with precise control ensures that this structure remains intact throughout the preparation process. Without this control, the resulting material would fail to meet the specifications required for its intended technological applications.
Understanding the Trade-offs
Sensitivity to Thermal Overshoot
The primary challenge in preparing beta-SiC is that the 1700°C limit acts as a hard ceiling. Even brief thermal spikes or "overshoots" in the heating equipment can initiate the transformation to the hexagonal phase.
Equipment Complexity
To maintain this precision, standard high-temperature furnaces may be insufficient. The process requires equipment capable of stable operation in the 1600°C range without fluctuating into the danger zone above 1700°C. This often necessitates sophisticated feedback loops and heating elements designed for high thermal uniformity.
Making the Right Choice for Your Goal
To ensure the successful preparation of Silicon Carbide, align your thermal strategy with your material requirements:
- If your primary focus is specific optical and electrical properties: Strictly maintain temperatures below 1700°C to preserve the cubic beta-SiC structure.
- If your primary focus is high-temperature structural stability: You may need to intentionally process above 1700°C to induce the transformation to the robust hexagonal phase.
Mastering the temperature ceiling is the single most important variable in synthesizing functional cubic beta-Silicon Carbide.
Summary Table:
| Feature | Beta-SiC (Cubic) | Hexagonal SiC |
|---|---|---|
| Crystal Structure | Zinc-blende (Cubic) | Hexagonal Lattice |
| Stability Range | Below 1700°C | Above 1700°C |
| Phase Transition | Desired for specific electronics | Irreversible transformation |
| Control Priority | Precise nucleation & thermal ceiling | High-temperature structural stability |
Achieve Perfection in beta-SiC Synthesis with KINTEK
Maintaining a strict thermal ceiling is the difference between high-performance beta-SiC and material failure. At KINTEK, we understand that even a minor thermal overshoot can ruin your cubic crystal lattice. Our laboratory solutions are engineered for the most demanding thermal management tasks.
Why partner with KINTEK?
- Unmatched Precision: Advanced feedback loops and heating elements designed for high thermal uniformity up to 1700°C.
- Customizable Systems: Whether you need a Vacuum, CVD, Muffle, or Tube furnace, our expert R&D team can tailor the system to your unique material specifications.
- Expert Manufacturing: Trusted by researchers and manufacturers for high-temp lab applications.
Ready to elevate your material science? Contact our experts today to find the perfect customizable furnace for your beta-SiC production.
Visual Guide
References
- Qingyuan Yu. Comparative Analysis of Sic and Gan: Third-Generation Semiconductor Materials. DOI: 10.54097/2q3qyj85
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- Why is an automatically controlled mesh belt quenching line used for bearing rings? Precision & Stability Explained
- How does the use of a stainless steel high-pressure autoclave affect ZnS/CeO2@CNT formation? Optimize Catalyst Growth
- What are the advantages of Spark Plasma Sintering (SPS)? Enhance Thermoelectric Performance in Copper Sulfide
- What is the significance of preheating UHPC molds? Ensure Safety & Longevity with High-Temp Furnaces
- What are the advantages of using a microwave reaction system? Rapid & Uniform Synthesis of Doped Hydroxyapatite
- What are the technical advantages of using the molten salt method? Elevate Your Biomass Carbon Support Synthesis
- What is the significance of maintaining an inert nitrogen atmosphere during molten salt activation? Ensure Pore Purity
- Why is it necessary to dry glassware in a 140 °C oven overnight before GTP? Ensure Precise Anhydrous Polymerization



















