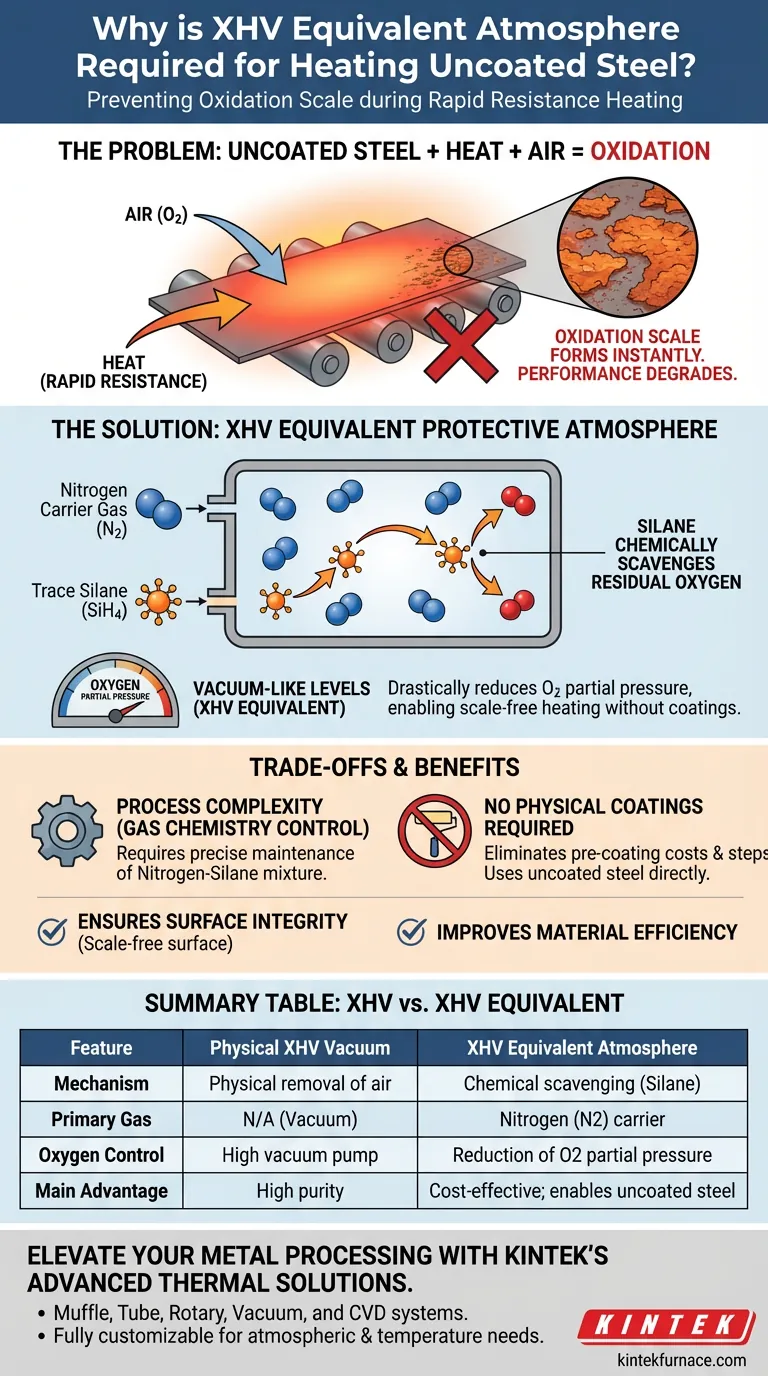
Uncoated steel sheets require an XHV (Extreme High Vacuum) equivalent atmosphere to completely prevent the rapid formation of oxidation scale during high-temperature resistance heating. Without this specialized environment, the steel reacts immediately with oxygen in the air, leading to surface degradation that compromises the final part's performance.
The core mechanism involves using trace silane (SiH4) within a nitrogen atmosphere to chemically scavenge residual oxygen. This reduces the oxygen partial pressure to levels comparable to an extreme high vacuum, enabling scale-free heating without the need for protective physical coatings.

The Chemistry of Oxidation Control
The Vulnerability of Uncoated Steel
When uncoated steel is subjected to rapid resistance heating, it is exposed to high temperatures in the presence of air. This environment causes oxidation scale to develop almost instantly on the surface of the sheet.
Why Scale Must Be Prevented
The formation of scale is not merely a cosmetic issue; it fundamentally degrades the performance of the manufactured part. To maintain the integrity of the steel, oxygen must be removed from the heating environment.
Achieving XHV Conditions Without a Vacuum
The Role of the "Equivalent" Atmosphere
Creating a physical Extreme High Vacuum (XHV) is mechanically complex and expensive. Instead, manufacturers can use an XHV equivalent protective atmosphere to achieve the same result chemically.
The Composition of the Atmosphere
This protective atmosphere consists primarily of nitrogen acting as a carrier gas. Crucially, it is mixed with trace amounts of silane (SiH4).
The Silane Reduction Mechanism
Silane is utilized for its strong chemical reduction properties. When introduced into the heating environment, the silane actively reacts with residual oxygen.
Lowering Oxygen Partial Pressure
This reaction drastically reduces the oxygen partial pressure within the chamber. By chemically eliminating the oxygen, the atmosphere mimics the purity of a physical vacuum, preventing oxidation from occurring.
Understanding the Trade-offs
Process Complexity vs. Physical Coatings
The primary trade-off in this approach is swapping the need for physical coatings for atmospheric control.
Eliminating Pre-Coating Requirements
Standard methods often require steel to be pre-coated to survive heating. Using an XHV equivalent atmosphere allows for the use of uncoated steel, streamlining material preparation.
Dependency on Gas Chemistry
Success relies entirely on the precise maintenance of the nitrogen-silane mixture. The process eliminates the vacuum pump but necessitates strict control over the chemical composition of the environment to ensure the reduction reaction is effective.
Making the Right Choice for Your Goal
This technology replaces physical barriers with chemical control to ensure surface purity.
- If your primary focus is Surface Integrity: Utilizing an XHV equivalent atmosphere ensures a scale-free surface by chemically reducing oxygen partial pressure to negligible levels.
- If your primary focus is Material Efficiency: This method allows you to process uncoated steel sheets directly, eliminating the costs and steps associated with applying protective physical coatings.
By leveraging the chemical reduction power of silane, you achieve the purity of a vacuum through the efficiency of atmospheric control.
Summary Table:
| Feature | Physical XHV Vacuum | XHV Equivalent Atmosphere |
|---|---|---|
| Mechanism | Physical removal of air molecules | Chemical scavenging using trace Silane (SiH4) |
| Primary Gas | N/A (Vacuum) | Nitrogen (N2) carrier |
| Oxygen Control | High vacuum pump extraction | Reduction of oxygen partial pressure |
| Main Advantage | High purity | Cost-effective; enables scale-free uncoated steel |
| Material Prep | None required | Eliminates need for protective coatings |
Elevate Your Metal Processing with KINTEK’s Advanced Thermal Solutions
Don't let oxidation compromise your material performance. KINTEK specializes in precision-engineered thermal systems designed for the most demanding industrial requirements. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to your specific atmospheric and temperature needs.
Whether you are processing uncoated steel sheets or developing advanced materials, our lab high-temp furnaces ensure the surface integrity your project demands. Contact KINTEK today to discuss your custom furnace requirements and see how our expertise can streamline your production and eliminate the need for costly pre-coatings.
Visual Guide
References
- Bernd‐Arno Behrens, Lorenz Albracht. Increasing the performance of hot forming parts by resistance heating in XHV-adequate atmosphere. DOI: 10.1051/matecconf/202540801025
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is it necessary to use an atmosphere furnace for MOF melt-quenching? Protect Fragile Materials from Decomposition
- What is the significance of nitrogen in atmosphere furnaces? Unlock Enhanced Heat Treatment and Surface Hardening
- How is atmosphere control managed during furnace operation? Master Precise Gas Environments for Superior Results
- What are some examples of inert gases used in inert atmospheres? Optimize Your Process with Nitrogen or Argon
- What factors should be considered when choosing a controlled atmosphere furnace? Ensure Optimal Performance for Your Materials
- What are the two main types of atmosphere furnaces based on design? Choose the Right Furnace for Your Lab
- Why is a continuous belt furnace with a controlled atmosphere required for sintering powder metallurgy steel?
- What are the advantages of using industrial-grade plasma nitriding furnaces? Boost Stainless Steel Surface Hardness



















