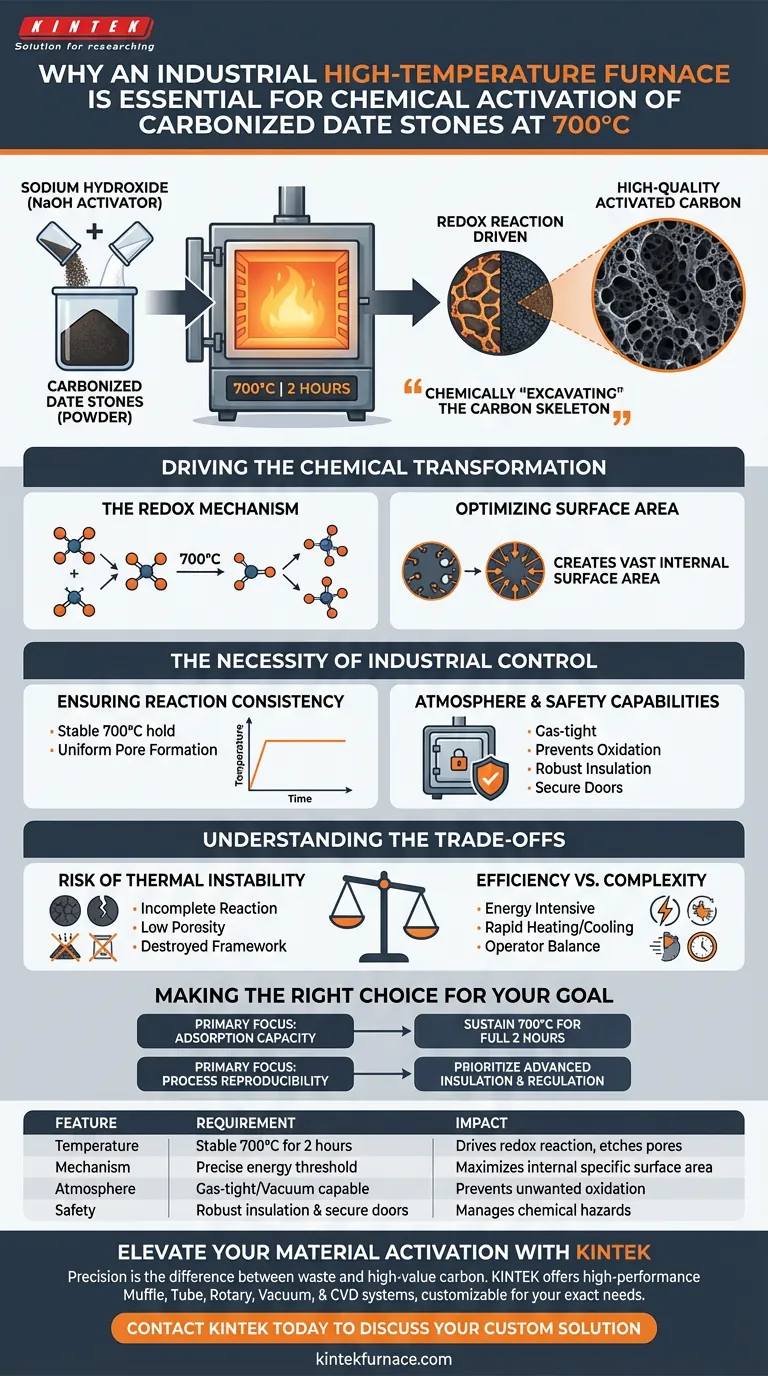
An industrial high-temperature furnace is strictly required to drive a specific redox reaction. To successfully activate carbonized date stones, you must maintain a consistent 700°C environment for exactly 2 hours. This thermal energy forces the sodium hydroxide chemical activator to react with the carbon matrix, physically altering the material's internal structure.
The furnace does not merely heat the material; it provides the precise energy threshold required to chemically "excavate" the carbon skeleton. This process creates the vast internal surface area that defines high-quality activated carbon.
Driving the Chemical Transformation
The Redox Mechanism
At ambient temperatures, the carbonized date stone powder and the chemical activator (sodium hydroxide) remain distinct.
You require a high-temperature environment of 700°C to induce a redox reaction. This reaction breaks down the dense carbon matrix, allowing the chemical agent to penetrate and restructure the material at a molecular level.
Optimizing Surface Area
The primary goal of this thermal treatment is the expansion of specific surface area.
As the redox reaction progresses, it etches pores into the carbon. This optimization of the pore structure is what transforms inert date stone powder into activated carbon with a high adsorption capacity.
The Necessity of Industrial Control
Ensuring Reaction Consistency
Chemical activation is time-dependent and temperature-sensitive.
According to your primary data, the process requires a stable hold at 700°C for 2 hours. An industrial furnace is designed to maintain this exact temperature without fluctuation, ensuring the activation agent fully penetrates the matrix to induce uniform pore formation.
Atmosphere and Safety Capabilities
High-performance furnaces, such as tubular models, offer necessary structural stability and gas-tightness.
This allows for precise atmosphere control, which prevents unwanted oxidation from outside air. Furthermore, industrial units provide essential safety features, such as robust insulation and secure door mechanisms, to manage the hazards of heating chemical agents to extreme temperatures.
Understanding the Trade-offs
The Risk of Thermal Instability
Using non-industrial or imprecise heating methods is a common point of failure.
If the temperature drops below 700°C, the redox reaction may remain incomplete, resulting in low porosity. Conversely, uncontrolled temperature spikes can destroy the carbon framework entirely, reducing yield.
Efficiency vs. Complexity
While effective, high-temperature activation is energy-intensive.
Industrial furnaces mitigate this through rapid heating and cooling cycles. However, the operator must balance the need for a thorough 2-hour soak time against the energy costs required to sustain high temperatures.
Making the Right Choice for Your Goal
To maximize the quality of your activated carbon, align your equipment use with your specific objective:
- If your primary focus is Adsorption Capacity: Ensure the furnace can sustain 700°C for the full 2-hour duration to maximize specific surface area expansion.
- If your primary focus is Process Reproducibility: Prioritize a furnace with advanced insulation and temperature regulation to guarantee identical pore structures across different batches.
Precision in the thermal environment is the single most critical factor in converting date stone waste into valuable activated carbon.
Summary Table:
| Feature | Requirement for Chemical Activation | Impact on Carbon Quality |
|---|---|---|
| Temperature | Stable 700°C for 2 hours | Drives the redox reaction to etch pores |
| Mechanism | Precise energy threshold | Maximizes internal specific surface area |
| Atmosphere | Gas-tight/Vacuum capable | Prevents unwanted oxidation and degradation |
| Safety | Robust insulation & secure doors | Manages chemical hazards at high heat |
Elevate Your Material Activation with KINTEK
Precision in thermal processing is the difference between waste and high-value activated carbon. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet your exact chemical activation needs.
Whether you are scaling production or refining lab-scale adsorption capacity, our industrial furnaces provide the temperature stability and atmosphere control required for consistent, high-yield results.
Ready to optimize your activation process? Contact KINTEK today to discuss your custom solution.
Visual Guide
References
- Nabil A. Alhemiary. Synthesis of Novel Nanocomposite CaO/AC/ZnO from Biogenic Wastes of Date Palm Seeds from The Najran Region (Saudi Arabia) and Eggshells for Degradation of Methylene Blue. DOI: 10.15379/ijmst.v11i1.3625
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is a muffle furnace and how does it generally function? Master Indirect Heating for Pure Results
- What task does a high-temperature box resistance furnace perform in Mg(Al1-xCrx)2O4 prep? Master Powder Calcination
- What is the role of a muffle furnace in the final formation of a composite photoanode? Master Heterojunction Synthesis
- What is the primary function of a high-temperature muffle furnace in graphene oxide synthesis? Maximize Carbon Yield
- What are the temperature and chamber size options for vacuum muffle furnaces? Find Your Perfect Fit for High-Temp Processes
- What are some major applications of muffle furnaces in research and industry? Unlock Precision Heat for Your Lab
- What are the limitations of crucible furnaces? Understand Key Trade-offs for Your Lab
- What are the key features of box furnaces? Versatile Thermal Solutions for Labs and Industry



















