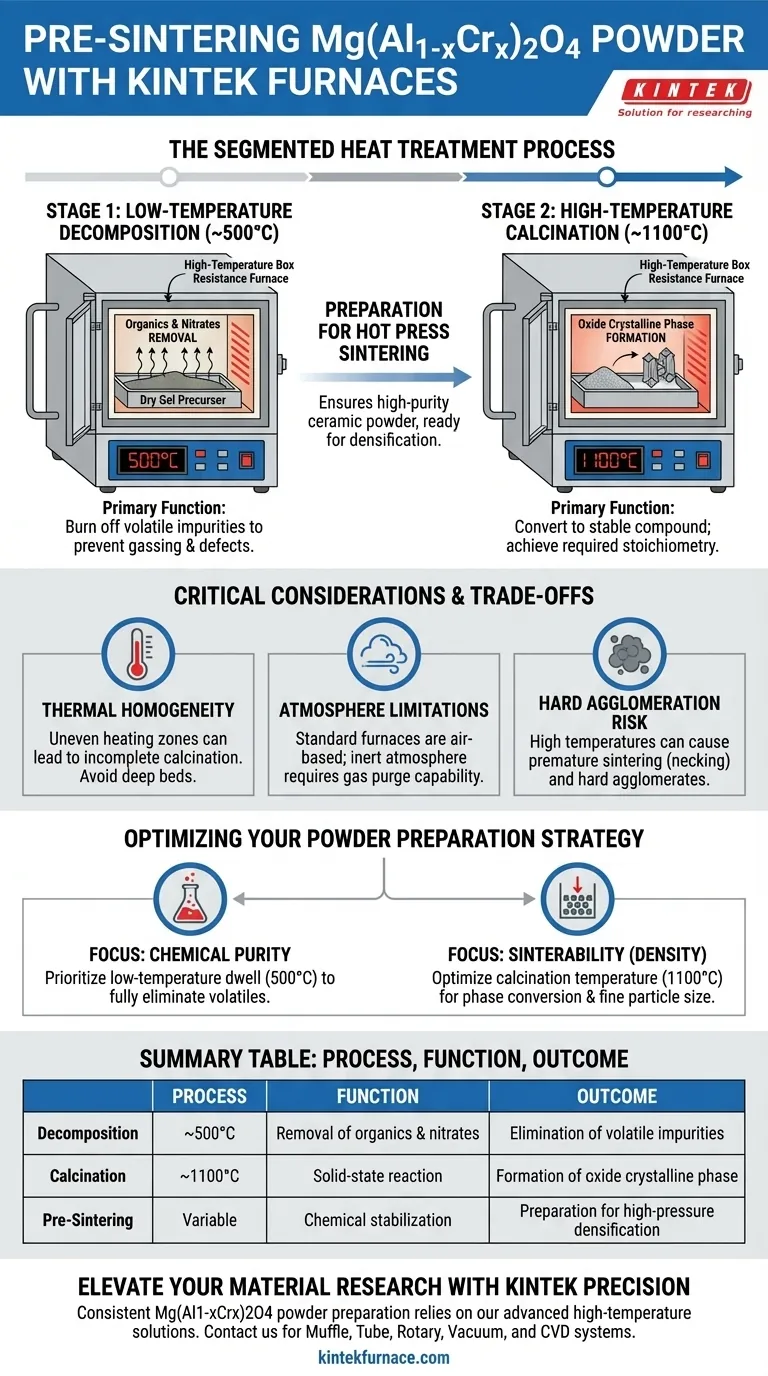
During the pre-sintering stage of Mg(Al1-xCrx)2O4 powder preparation, the high-temperature box resistance furnace performs a precise, segmented heat treatment. Its primary function is to transform the raw "dry gel" precursor into a stable, high-purity ceramic powder through a two-step thermal process: first holding at lower temperatures (e.g., 500°C) to decompose organic impurities, and then ramping to high temperatures (e.g., 1100°C) to crystallize the final oxide phase.
Core Takeaway The furnace serves not just as a heater, but as a chemical reactor that purifies and stabilizes the material. Its role is to fully eliminate volatile components (organics and nitrates) and establish the correct crystalline structure, ensuring the powder is chemically ready for the subsequent high-pressure densification processes.

The Mechanics of the Segmented Process
The "pre-sintering" or powder preparation stage is distinct from the final densification. It focuses on chemical conversion rather than physical shape-forming. The box furnace (muffle furnace) achieves this through two distinct thermal plateaus.
Stage 1: Low-Temperature Decomposition
The first task is the removal of chemical byproducts remaining from the precursor synthesis. The furnace holds the material at a moderate temperature, typically around 500°C.
At this stage, the heat drives the decomposition of organics and nitrates trapped within the dry gel. This step is critical; if these volatiles are not removed here, they will cause gassing and structural defects during the final high-temperature sintering.
Stage 2: High-Temperature Calcination
Once impurities are burned off, the furnace raises the temperature significantly, often reaching 1100°C.
This is the calcination phase. The thermal energy triggers a solid-state reaction that converts the amorphous or intermediate precursor material into a definite oxide crystalline phase. This ensures the powder achieves the specific chemical stoichiometry required for the Mg(Al1-xCrx)2O4 compound.
Preparation for Hot Press Sintering
The output of this furnace cycle is a high-purity ceramic powder. By completing phase transformation and volatile elimination before the material enters a hot press, the furnace ensures the final sintering step can focus solely on densification and grain growth without the interference of chemical off-gassing.
Critical Considerations and Trade-offs
While the box resistance furnace is the standard tool for this task, understanding its limitations is essential for consistent results.
Thermal Homogeneity
Box furnaces rely on radiative heating from resistive elements. A common pitfall is uneven heating zones within the chamber.
If the powder bed is too deep or placed near the door, portions of the batch may not reach the target 1100°C. This results in "incomplete calcination," leaving behind unstable phases that will degrade the performance of the final ceramic part.
Atmosphere Limitations
Standard muffle furnaces typically operate under atmospheric pressure (air).
For oxide ceramics like Mg(Al1-xCrx)2O4, this is generally beneficial as it promotes oxidation. However, if your specific doping formulation requires an inert atmosphere to prevent the oxidation of specific transition metals, a standard box furnace may act as a contaminant source unless equipped with gas purge capabilities.
The Risk of Hard Agglomeration
High calcination temperatures (1100°C) improve purity but can lead to coarsening.
If the temperature is held too long or goes too high, the powder particles may begin to sinter prematurely (necking) into hard agglomerates. These agglomerates are difficult to break down and can create voids in the final hot-pressed product.
Optimizing Your Powder Preparation Strategy
To ensure the highest quality Mg(Al1-xCrx)2O4 powder, tailor your furnace usage to your specific constraints.
- If your primary focus is Chemical Purity: Prioritize the low-temperature dwell time (500°C). Ensure the hold is long enough to fully burn off all nitrates and organics to prevent bloating later.
- If your primary focus is Sinterability (Density): Carefully optimize the calcination temperature (1100°C). Aim for the lowest temperature that achieves full phase conversion to keep the particle size fine and reactive.
Success lies in treating this furnace run as a precise chemical synthesis step, not just a drying process.
Summary Table:
| Process Stage | Temperature | Primary Function | Key Outcome |
|---|---|---|---|
| Decomposition | ~500°C | Removal of organics & nitrates | Elimination of volatile impurities |
| Calcination | ~1100°C | Solid-state reaction | Formation of oxide crystalline phase |
| Pre-Sintering | Variable | Chemical stabilization | Preparation for high-pressure densification |
Elevate Your Material Research with KINTEK Precision
Consistency in Mg(Al1-xCrx)2O4 powder preparation depends on the thermal precision and reliability of your equipment. KINTEK provides industry-leading high-temperature solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all engineered to deliver the uniform heating required for complex calcination and sintering processes.
Backed by expert R&D and advanced manufacturing, our laboratory furnaces are fully customizable to meet your unique chemical synthesis and material science needs. Ensure your research achieves maximum purity and optimal densification—Contact KINTEK today for a custom furnace solution!
Visual Guide
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is re-calcination in a muffle furnace necessary for photocatalysts? Restore Efficiency via Thermal Oxidation
- What temperature range can muffle furnaces typically operate within? Find the Perfect Fit for Your Lab
- What role does a box muffle furnace play during the pre-carbonization stage of sugarcane bagasse? Expert Insights
- What are the key disadvantages of a muffle furnace? Slow cycles, high energy use, and maintenance challenges
- How do box type resistance furnaces contribute to catalytic material preparation? Unlock Precision in Catalyst Synthesis
- What is the use of muffle furnace in laboratory? Achieve Pure, High-Temperature Heat for Accurate Analysis
- What is a Muffle Furnace with Hydrogen atmosphere? Achieve Oxide-Free, Bright Metal Finishes
- How does a muffle furnace ensure temperature uniformity? Discover the Key to Precise Heat Control



















