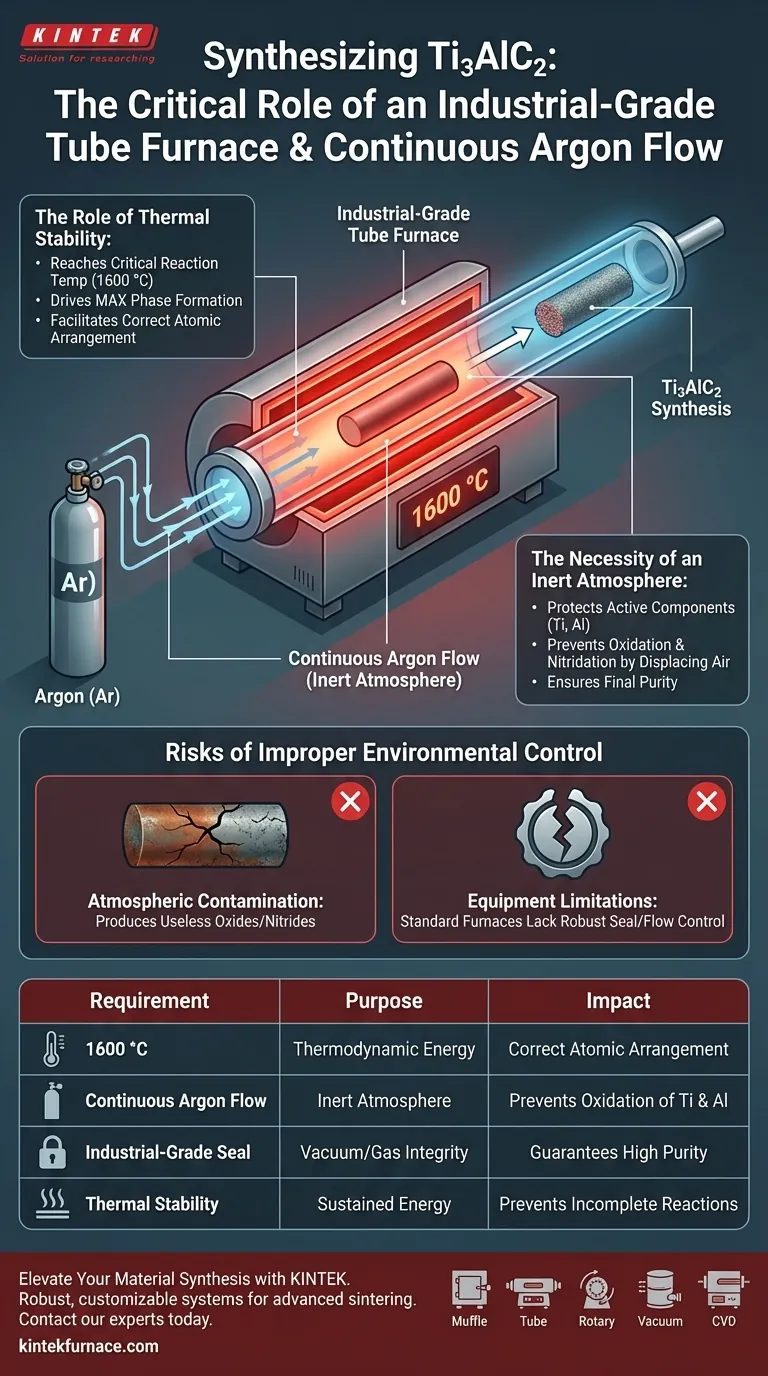
To successfully synthesize Ti3AlC2, you must maintain a strictly controlled thermal and chemical environment. An industrial-grade tube furnace is required to provide the stable 1600 °C temperature necessary for phase formation, while a continuous argon flow prevents the rapid degradation of reactive elements like titanium and aluminum.
Synthesis of MAX phases like Ti3AlC2 relies on a delicate balance of extreme heat and chemical isolation. The equipment setup is not just about reaching a temperature; it is about sustaining that energy in a vacuum of potential contaminants to ensure the material forms correctly.

The Role of Thermal Stability
Reaching Critical Reaction Temperatures
The synthesis of Ti3AlC2 is a high-energy process. You require an industrial-grade tube furnace capable of reaching and sustaining a temperature of 1600 °C.
Driving the MAX Phase Formation
This specific temperature threshold is non-negotiable. It provides the necessary thermodynamic energy to drive the reaction between the raw materials, facilitating the correct atomic arrangement of the MAX phase.
The Necessity of an Inert Atmosphere
Protecting Active Components
At 1600 °C, the raw materials used in this synthesis—specifically titanium and aluminum—become highly "active." In this state, they are extremely susceptible to reacting with the surrounding environment.
Preventing Oxidation and Nitridation
Without protection, these active components would immediately react with oxygen or nitrogen in the air. A continuous flow of argon gas floods the tube, displacing air and creating an inert atmosphere.
Ensuring Final Purity
By eliminating the possibility of oxidation or nitridation, the argon flow ensures that the titanium and aluminum react only with each other and the carbon source. This is the only way to guarantee the purity of the synthesized Ti3AlC2.
Risks of Improper Environmental Control
The Cost of Atmospheric Contamination
If the argon flow is interrupted or the furnace seal is compromised, the high reactivity of the components will lead to immediate failure. Instead of pure Ti3AlC2, you will likely produce useless oxides or nitrides.
Equipment Limitations
Standard furnaces often lack the ability to maintain a strictly controlled gas flow at 1600 °C. The designation of "industrial-grade" implies the robustness required to maintain both thermal stability and a hermetic gas seal over the duration of the sintering process.
Making the Right Choice for Your Goal
When setting up your synthesis protocol, prioritize equipment that offers precise control over both heat and atmosphere.
- If your primary focus is Phase Formation: Ensure your furnace can reliably sustain 1600 °C without fluctuation to drive the reaction.
- If your primary focus is Material Purity: Verify that your system supports a continuous argon flow to completely shield the active titanium and aluminum components.
Control the environment, and you control the quality of your Ti3AlC2.
Summary Table:
| Requirement | Purpose in Ti3AlC2 Synthesis | Impact on Final Material |
|---|---|---|
| 1600 °C Temperature | Provides thermodynamic energy for MAX phase formation | Ensures correct atomic arrangement |
| Continuous Argon Flow | Creates an inert atmosphere to displace oxygen/nitrogen | Prevents oxidation and nitridation of Ti and Al |
| Industrial-Grade Seal | Maintains vacuum/gas integrity at high heat | Guarantees high purity and prevents phase failure |
| Thermal Stability | Sustains high energy throughout sintering | Prevents incomplete reactions or secondary phases |
Elevate Your Material Synthesis with KINTEK
Precise control over thermal and chemical environments is the difference between a pure MAX phase and a failed experiment. KINTEK provides the robust, high-performance equipment needed for advanced sintering. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique laboratory needs.
Whether you are synthesizing Ti3AlC2 or developing next-generation ceramics, our industrial-grade tube furnaces ensure the 1600 °C stability and hermetic gas control your research demands.
Ready to optimize your high-temperature processes? Contact our experts today to find the perfect furnace solution for your lab.
Visual Guide
References
- Karamullah Eisawi, Michael Naguib. Nanohybrid of Silver‐MXene: A Promising Sorbent for Iodine Gas Capture from Nuclear Waste. DOI: 10.1002/admi.202500011
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is a tube furnace and what is its primary use? Achieve Precision High-Temp Processing for Your Lab
- What is the purpose of using a tube furnace for a second calcination at 750°C? Mastering Biochar Activation
- What role does a quartz tube furnace play in the carbonization of nitrogen-doped carbon? Optimize Your Material Synthesis
- What is the significance of a multi-zone configuration in a horizontal tube furnace? Master FC-CVD Synthesis Control
- What are some advanced features of more elaborate tube furnaces? Unlock Precision Control for High-Temp Processes
- How does a vacuum tube furnace function in Ti6Al4V post-processing? Optimize Additive Manufacturing Outcomes
- What is the function of a high-temperature tube furnace in Cu(111) transformation? Achieve Atomic Precision
- How have tube furnaces evolved over time? From Basic Heating to Precision Control



















