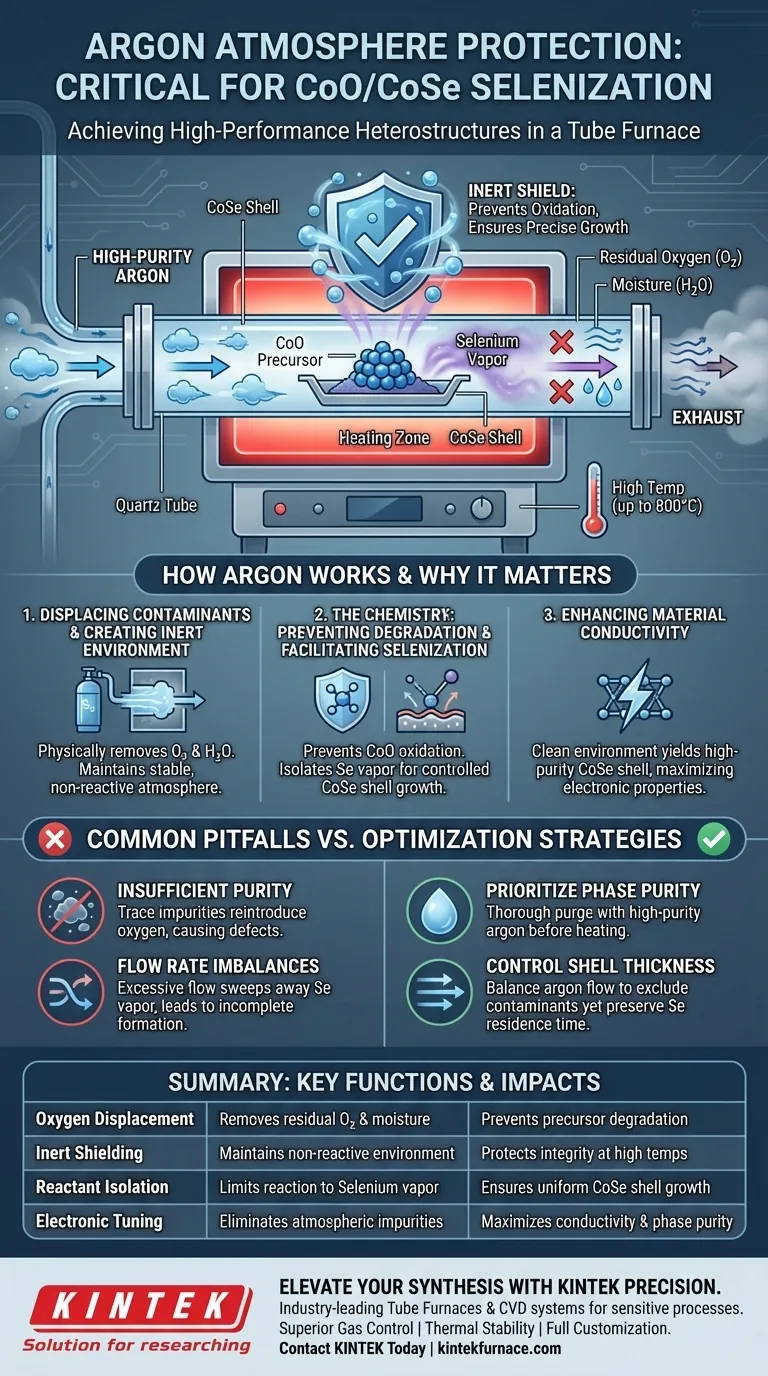
The use of an argon atmosphere is strictly required to create a chemically inert environment during the high-temperature selenization of CoO/CoSe heterostructures. By displacing oxygen and moisture within the tube furnace, high-purity argon prevents the uncontrolled oxidation of cobalt precursors while ensuring the precise, uniform formation of the conductive cobalt selenide (CoSe) shell.
Argon acts as a critical shield, protecting the reaction from atmospheric contaminants that would degrade the material. This controlled environment ensures the synthesis of a high-conductivity CoO/CoSe core-shell heterostructure with optimal structural integrity.

Mechanisms of Atmosphere Control
Displacing Contaminants
The primary function of the argon flow is the physical removal of reactive elements from the furnace chamber. Before the temperature rises, argon pushes out residual oxygen and ambient moisture that naturally exist within the tube.
Creating an Inert Environment
Once contaminants are removed, argon maintains a stable, non-reactive atmosphere throughout the process. This allows the system to reach high processing temperatures (specifically 800°C) without initiating unwanted chemical side reactions.
The Chemistry of Heterostructure Formation
Preventing Precursor Degradation
Cobalt-based materials are highly susceptible to oxidation when exposed to high heat. Without argon protection, the precursor would likely degrade into unwanted oxides rather than maintaining the stable Cobalt Oxide (CoO) core necessary for the heterostructure.
Facilitating Precise Selenization
The inert atmosphere ensures that selenium vapor is the only active reactant interacting with the precursor surface. This isolation allows for the controlled growth of the CoSe shell, rather than a chaotic mixture of oxides and selenides.
Enhancing Material Conductivity
A clean reaction environment directly contributes to the electronic properties of the final material. The formation of a high-purity CoSe shell is essential for achieving the high conductivity required for high-performance applications.
Common Pitfalls to Avoid
Insufficient Purity Levels
The protection offered by the atmosphere is only as good as the gas source. Using argon with trace impurities can reintroduce oxygen into the system, leading to surface defects even at optimal temperatures.
Flow Rate Imbalances
While argon is essential for protection, the flow rate must be carefully balanced. Excessive gas flow can potentially sweep away selenium vapor too quickly, resulting in incomplete shell formation or uneven coating.
Optimizing Your Synthesis Parameters
Achieving a perfect core-shell structure requires balancing gas purity with precise temperature management.
- If your primary focus is phase purity: Ensure the tube furnace is thoroughly purged with high-purity argon prior to heating to eliminate all traces of moisture and oxygen.
- If your primary focus is shell thickness control: Maintain a steady argon flow that excludes contaminants but preserves the necessary residence time for selenium vapor at the precursor surface.
Rigorous atmosphere control is the defining factor between a degraded sample and a high-performance heterostructure.
Summary Table:
| Key Function | Mechanism | Impact on CoO/CoSe Heterostructure |
|---|---|---|
| Oxygen Displacement | Removes residual O2 and moisture | Prevents degradation of cobalt precursors into unwanted oxides |
| Inert Shielding | Maintains non-reactive environment | Protects material integrity at high temperatures (up to 800°C) |
| Reactant Isolation | Limits reaction to selenium vapor | Ensures uniform growth of the conductive CoSe shell |
| Electronic Tuning | Eliminates atmospheric impurities | Maximizes conductivity and phase purity for high-performance use |
Elevate Your Material Synthesis with KINTEK Precision
Don’t let atmospheric contamination compromise your research. KINTEK provides industry-leading Tube Furnaces and CVD systems designed specifically for sensitive processes like selenization.
Backed by expert R&D and manufacturing, our systems offer:
- Superior Gas Control: Optimized for argon purging to eliminate oxygen and moisture.
- Thermal Stability: Precise temperature management for uniform heterostructure formation.
- Full Customization: Tailored solutions for Muffle, Rotary, and Vacuum systems to meet your unique lab requirements.
Contact KINTEK Today to consult with our experts and secure the perfect high-temperature solution for your next breakthrough.
Visual Guide
References
- Shasha Song, Xingqun Zhu. Synthesis and Lithium Storage Performance of CoO/CoSe Composite Nanoparticles Supported on Carbon Paper. DOI: 10.54691/k2djhp47
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What materials besides metals benefit from inert atmosphere heat treating? Protect High-Performance Polymers Like PTFE
- What types of configurations are available for retort furnaces? Optimize Your Thermal Process with the Right Setup
- What safety features are included in the box type annealing atmosphere furnace? Ensure Operator and Equipment Protection
- Why is a high-purity argon protection system required for CP-Ti? Protect Ductility in Titanium Heat Treatment
- How does atmosphere control affect defect formation in graphitic carbon nitride? Master Atmosphere Engineering
- Why are vacuum or atmosphere control systems required for Fe, Co, and Ni single-atom catalysts? Ensure Atomic Precision
- How are atmosphere furnaces applied in the preparation of optical materials? Enhance Clarity and Performance
- What are the key functions of furnace atmospheres in heat treatment? Master Protective and Active Roles



















