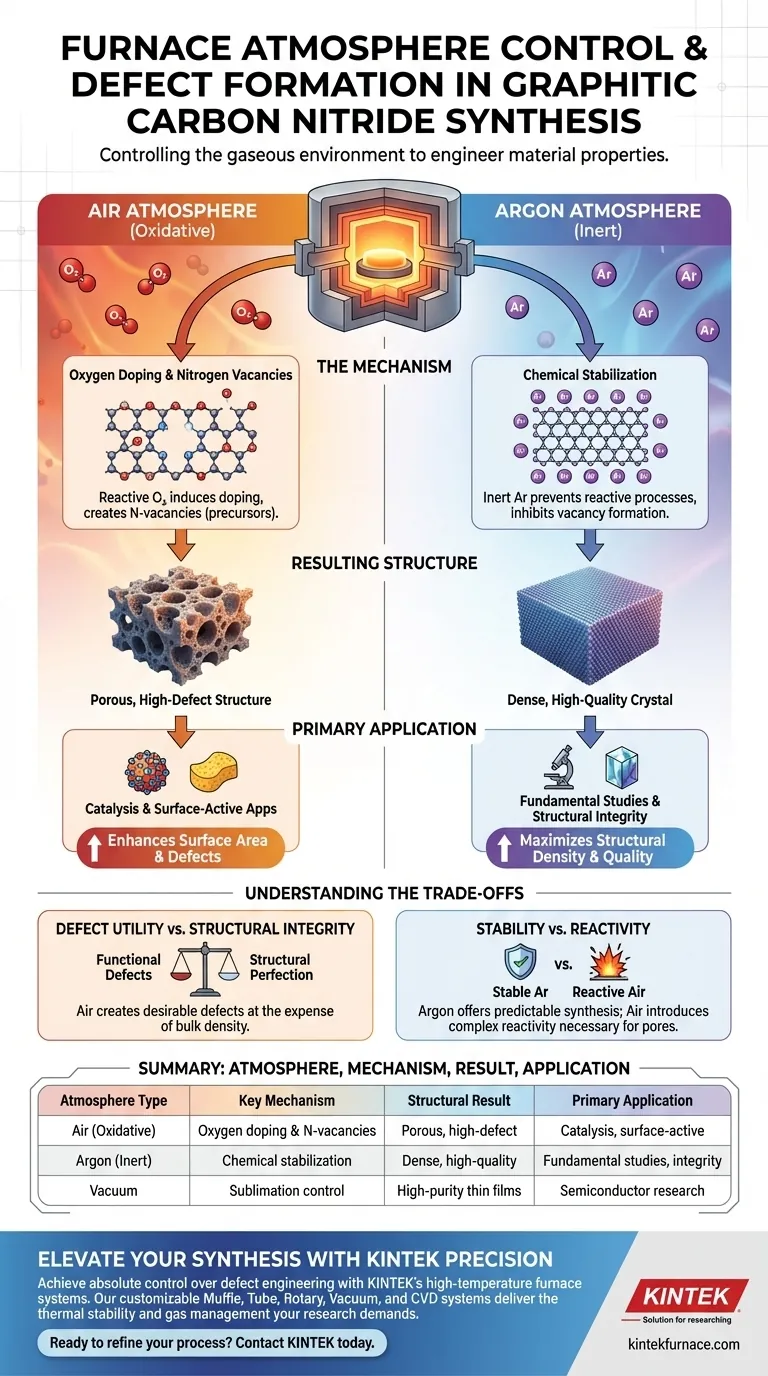
The gaseous environment within a high-temperature furnace serves as the primary control lever for determining the defect density in synthesized graphitic carbon nitride. When synthesis occurs in an air atmosphere, the presence of oxygen induces doping and creates nitrogen vacancies, which act as critical precursors for porous defect formation. Conversely, utilizing an inert argon atmosphere prevents these reactive processes, yielding a denser material structure with minimal defects.
Controlling the furnace atmosphere allows you to switch between creating a high-density crystal and a highly defective, porous material. While inert gases preserve structural integrity, an air atmosphere is required to induce the specific oxygen doping and nitrogen vacancies necessary for subsequent pore formation.

The Mechanism of Defect Formation
The Role of an Air Atmosphere
In an air environment, oxygen is an active participant in the synthesis process. It does not merely surround the sample; it chemically interacts with the developing lattice. This interaction induces oxygen doping within the graphitic carbon nitride structure.
Creating Precursors for Porosity
The most significant impact of air synthesis is the generation of nitrogen vacancies. These vacancies are not static; they serve as chemical precursors. During subsequent etching stages, these specific defect sites evolve into in-plane porous defects.
The Role of an Inert Atmosphere
When an argon atmosphere is used, the synthesis environment is rendered chemically inert. There are no reactive gases present to induce doping or strip nitrogen from the lattice. This effectively shuts down the mechanism responsible for vacancy formation.
Resulting Structural Density
Because the inert atmosphere prevents the formation of defect precursors, the resulting material differs fundamentally from air-synthesized counterparts. The final product is structurally denser. It creates a baseline material with significantly fewer crystalline imperfections.
Understanding the Trade-offs
Defect Utility vs. Structural Integrity
Choosing an atmosphere is a trade-off between functional defects and structural perfection. An air atmosphere creates defects that may be desirable for catalytic activity or surface area. However, this comes at the expense of the material's bulk density and crystalline order.
Stability vs. Reactivity
Argon atmospheres provide a stable, predictable synthesis route ideal for fundamental studies. Air introduces reactivity that complicates the material chemistry. While this reactivity is necessary for pore formation, it requires precise control to avoid degrading the material beyond utility.
Making the Right Choice for Your Synthesis Goal
To select the appropriate furnace atmosphere, you must define the desired attributes of your final material.
- If your primary focus is enhancing surface area and creating porous defects: Utilize an air atmosphere to induce oxygen doping and nitrogen vacancies.
- If your primary focus is obtaining a dense, high-quality crystal structure: Utilize an argon atmosphere to minimize reactive interference and defect formation.
By strategically alternating between oxidative and inert atmospheres, you transition from passive synthesis to active defect engineering.
Summary Table:
| Atmosphere Type | Key Mechanism | Structural Result | Primary Application |
|---|---|---|---|
| Air (Oxidative) | Oxygen doping & nitrogen vacancies | Porous, high-defect structure | Catalysis & surface-active apps |
| Argon (Inert) | Chemical stabilization | Dense, high-quality crystal | Fundamental studies & structural integrity |
| Vacuum | Sublimation control | High-purity thin films | Semiconductor research |
Elevate Your Material Synthesis with Precision Atmosphere Control
Precise defect engineering in graphitic carbon nitride requires the absolute control provided by KINTEK’s high-temperature furnace systems. Whether you are inducing nitrogen vacancies in an oxidative environment or preserving crystalline density under argon, our equipment delivers the thermal stability and gas management your research demands.
Backed by expert R&D and manufacturing, KINTEK offers customizable Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to the unique needs of material scientists and lab professionals.
Ready to refine your synthesis process? Contact us today to find the perfect high-temp solution for your lab.
Visual Guide
References
- New Insights In‐Plane Porous Defects Formation Mechanism of Single‐Layer Graphitic Carbon Nitride by Tetrahydrofuran Etching Reaction. DOI: 10.1002/sstr.202500259
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does the coordination between a heating furnace and a rolling mill contribute to processing high-entropy alloys?
- What is the purpose of an atmosphere furnace? Control Gas Environments for Superior Material Processing
- Which industries benefit from the versatility of retort furnaces? Unlock Precise Heat and Atmosphere Control
- What are the key advantages of using atmosphere furnaces? Boost Efficiency and Control in Heat Treatment
- What is a box type annealing atmosphere furnace? Master Controlled Heat Treatment for Superior Materials
- Why is an XHV equivalent protective atmosphere required for heating uncoated steel? Achieve Scale-Free Surface Purity
- What is the core difference between box and atmosphere furnaces? Choose the Right Equipment for Your Lab
- What role does atmosphere control play in ruthenium loading for catalyst synthesis? Master Precision Kinetics



















