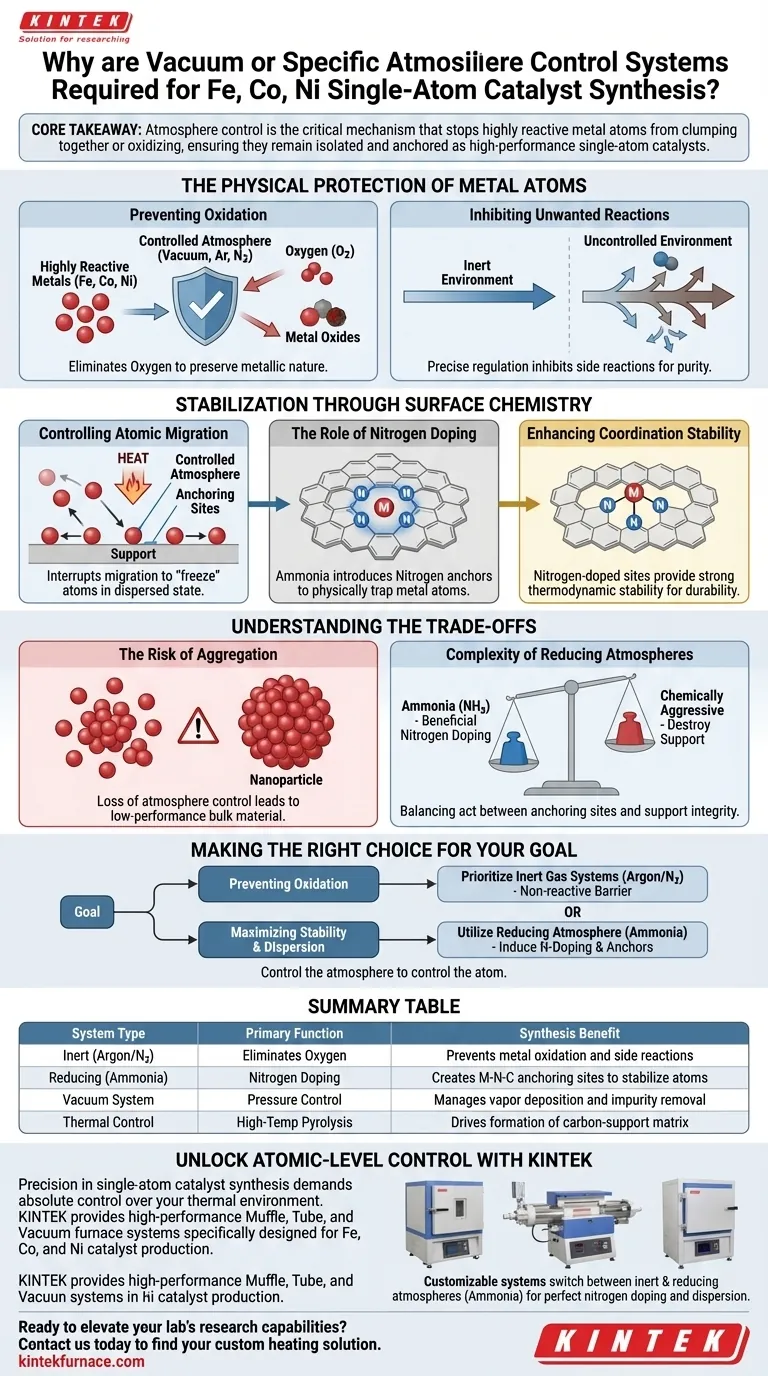
Vacuum or specific atmosphere control systems are strictly required to precisely regulate the pyrolysis environment during the synthesis of Iron (Fe), Cobalt (Co), and Nickel (Ni) single-atom catalysts. These systems utilize gases like argon, nitrogen, or ammonia to prevent the metal atoms from reacting with oxygen or migrating to form bulk nanoparticles at the elevated temperatures necessary for synthesis.
Core Takeaway: Atmosphere control is the critical mechanism that stops highly reactive metal atoms from clumping together or oxidizing, ensuring they remain isolated and anchored as high-performance single-atom catalysts.

The Physical Protection of Metal Atoms
Preventing Oxidation
Fe, Co, and Ni are highly reactive transition metals. At the high temperatures required for pyrolysis, these metals will instantly react with any available oxygen to form metal oxides. Atmosphere control systems eliminate oxygen from the chamber to preserve the metallic nature or specific coordination of the atoms.
Inhibiting Unwanted Reactions
Beyond oxidation, uncontrolled environments can lead to unpredictable chemical side reactions. By using vacuum or inert gas flows, you inhibit these undesirable reactions that compromise the purity of the catalyst. This precise regulation ensures that the thermal energy is used solely for the intended synthesis pathways.
Stabilization Through Surface Chemistry
Controlling Atomic Migration
Heat provides metal atoms with kinetic energy, causing them to move across the support surface. Without intervention, these atoms naturally migrate and aggregate into larger clusters or nanoparticles to lower their surface energy. Controlled atmospheres are essential to interrupt this migration and "freeze" the atoms in a dispersed state.
The Role of Nitrogen Doping
A specific reducing atmosphere, such as ammonia, plays a dual role by facilitating nitrogen doping. Nitrogen atoms introduced into the carbon support act as "anchors" for the metal atoms. This creates stable coordination sites that physically trap the Fe, Co, or Ni atoms, preventing them from moving.
Enhancing Coordination Stability
The stability of a single-atom catalyst depends on how well the metal atom is bonded to its support. The nitrogen-doped sites created under these controlled atmospheres provide the strongest thermodynamic stability for single atoms. This ensures the catalyst remains durable and active even during harsh operating conditions.
Understanding the Trade-offs
The Risk of Aggregation
The most significant risk in these systems is the formation of nanoparticles. If the atmosphere control fails or the gas composition is incorrect, the "anchoring" effect is lost. The metal atoms will immediately aggregate, converting the high-efficiency single-atom catalyst into a standard, lower-performance bulk material.
Complexity of Reducing Atmospheres
While ammonia promotes beneficial nitrogen doping, it is chemically aggressive. Using a reducing atmosphere requires precise calibration to ensure it modifies the support without destroying the underlying structure. It is a balancing act between creating anchoring sites and maintaining the integrity of the carbon matrix.
Making the Right Choice for Your Goal
- If your primary focus is preventing oxidation: Prioritize inert gas systems (Argon or Nitrogen) to create a strictly non-reactive barrier against oxygen.
- If your primary focus is maximizing stability and dispersion: Utilize a reducing atmosphere (Ammonia) to induce nitrogen doping and create robust anchoring sites for the metal atoms.
Control the atmosphere to control the atom.
Summary Table:
| System Type | Primary Function | Synthesis Benefit |
|---|---|---|
| Inert (Argon/N2) | Eliminates Oxygen | Prevents metal oxidation and side reactions |
| Reducing (Ammonia) | Nitrogen Doping | Creates M-N-C anchoring sites to stabilize atoms |
| Vacuum System | Pressure Control | Manages vapor deposition and impurity removal |
| Thermal Control | High-Temp Pyrolysis | Drives the formation of the carbon-support matrix |
Unlock Atomic-Level Control with KINTEK
Precision in single-atom catalyst synthesis demands absolute control over your thermal environment. KINTEK provides high-performance Muffle, Tube, and Vacuum furnace systems specifically designed for the rigorous demands of Fe, Co, and Ni catalyst production.
Backed by expert R&D and world-class manufacturing, our customizable systems allow you to seamlessly switch between inert and reducing atmospheres (like Ammonia) to ensure perfect nitrogen doping and prevent nanoparticle aggregation.
Ready to elevate your lab's research capabilities? Contact us today to find your custom heating solution and experience the KINTEK advantage in material science.
Visual Guide
References
- Yuquan Yang, Jinlong Zheng. Preparation of Fe, Co, Ni-based single atom catalysts and the progress of their application in electrocatalysis. DOI: 10.20517/microstructures.2024.65
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How is the protective atmosphere box furnace utilized in powder metallurgy? Essential for Sintering Metal Powders
- What are inert gas atmospheres and how are they used in heat treatment? Prevent Oxidation and Decarburization for Superior Metal Parts
- How is an atmosphere furnace used in material science research? Unlock Precise Material Control and Synthesis
- What are the technical advantages of a Zero-reforming Vertical Furnace? Revolutionize Green DRI Production Today
- What is the relationship between temperature and the furnace atmosphere in material processing? Master the Critical Heat-Environment Balance
- What process conditions does a box annealing furnace provide for Ti50Ni47Fe3 alloy? Optimize Heat Treatment Parameters
- Why is a uniform atmosphere important in carburizing workpieces? Ensure Consistent Hardness and Prevent Failures
- What are the safety and operational requirements for box furnaces and atmosphere furnaces? Ensure Safe, Efficient Heat Treatment



















