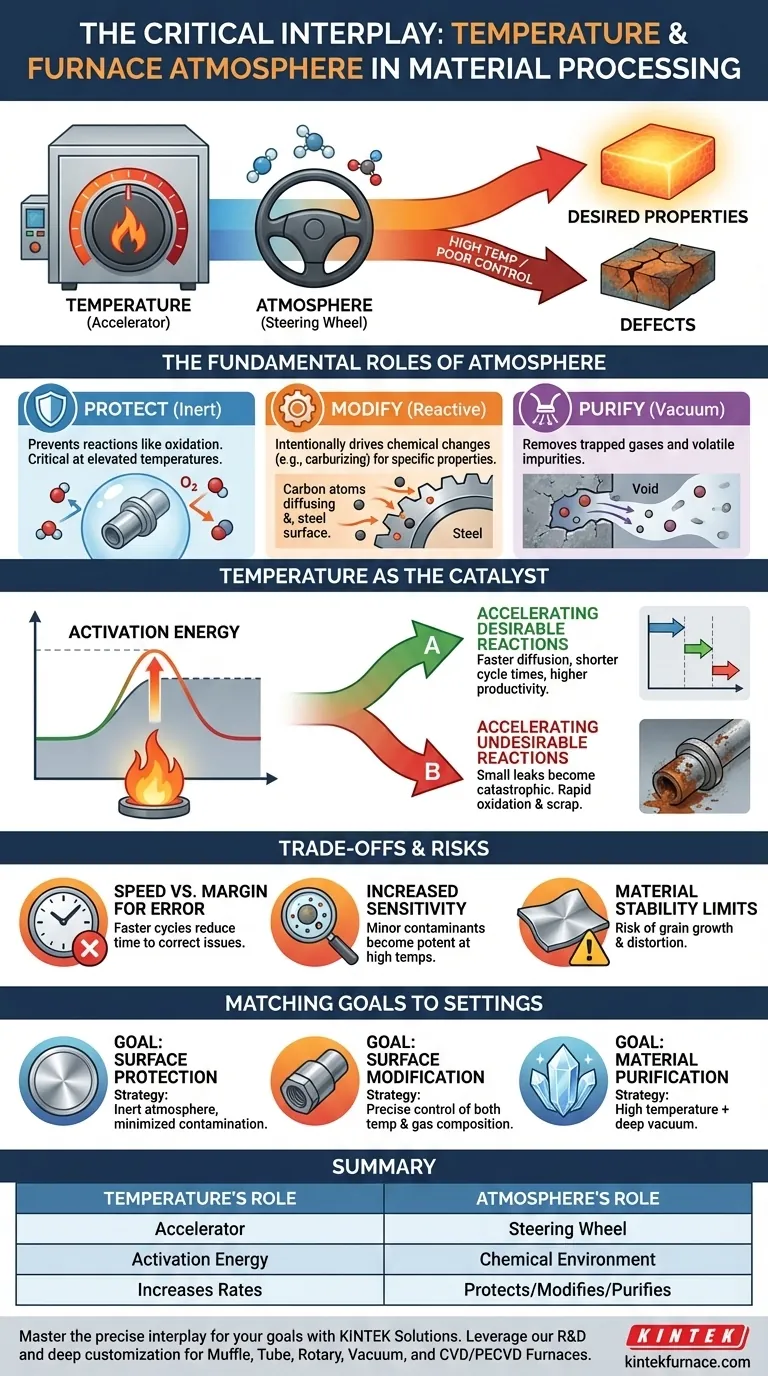
In material processing, temperature and furnace atmosphere are not independent variables; they are a deeply interconnected system. The relationship is direct and critical: temperature acts as a catalyst, dramatically amplifying the effects—both positive and negative—of the atmosphere surrounding a material. As temperatures rise, the rate of all chemical reactions increases, making precise atmospheric control essential for achieving the desired outcome.
Think of temperature as the accelerator and the furnace atmosphere as the steering wheel. As you increase the temperature, the process moves faster, making precise control of the atmosphere absolutely critical to steer the material toward its desired properties and away from defects.

The Fundamental Roles of a Furnace Atmosphere
The purpose of a controlled atmosphere is to dictate the chemical environment at the material's surface. This environment can be broadly categorized into two primary functions, both of which are heavily influenced by temperature.
To Protect the Material
An inert atmosphere is designed to be chemically non-reactive with the material. Gases like argon or nitrogen are used to displace oxygen and moisture.
The primary goal here is prevention. At elevated temperatures, most metals will readily react with oxygen to form oxides (scale), which can ruin the surface finish and dimensional accuracy of a part.
To Modify the Material
A reactive atmosphere is intentionally chosen to cause a specific chemical change on the material's surface.
Processes like carburizing, for example, use a carbon-rich atmosphere at high temperatures to diffuse carbon atoms into the surface of steel, hardening it. Here, the atmosphere is an active ingredient in the process.
To Purify the Material
A vacuum atmosphere is the absence of a conventional atmosphere. At high temperatures, a vacuum can effectively pull unwanted trapped gases and other volatile impurities out of the material itself.
This is crucial in applications like brazing or sintering advanced materials where internal purity is paramount for performance.
How Temperature Activates the Atmosphere
Temperature is the energy that drives the interactions between the atmosphere and the material. Without sufficient heat, many of these crucial reactions would happen too slowly to be practical, or not at all.
The Concept of Activation Energy
Every chemical reaction requires a certain amount of energy to get started, known as activation energy. Heat provides this energy.
Increasing the furnace temperature gives more atoms the necessary energy to react, dramatically speeding up the entire process.
Accelerating Desirable Reactions
In a surface modification process like carburizing, higher temperatures allow carbon to diffuse into the steel much faster. This directly translates to shorter cycle times and higher productivity.
The relationship is predictable, allowing engineers to use temperature as a primary control for determining the depth of the hardened case.
Accelerating Undesirable Reactions
The same principle applies to unwanted reactions. If an inert atmosphere intended for annealing has a small oxygen leak, this contamination may be harmless at low temperatures.
At high temperatures, however, this small amount of oxygen becomes highly reactive, rapidly causing heavy oxidation and potentially scrapping an entire batch of parts.
Understanding the Trade-offs and Risks
While higher temperatures can increase efficiency, they also introduce significant risks and require more stringent process control.
The Double-Edged Sword of Speed
Faster cycle times are economically desirable. However, this speed reduces the margin for error.
An imbalance in the furnace atmosphere that might take an hour to cause a minor issue at a lower temperature could cause a catastrophic failure in minutes at a higher temperature.
Increased Sensitivity to Contaminants
High temperatures make processes far more sensitive to impurities in the atmosphere.
A tiny amount of moisture or a trace gas that would be negligible at 500°C can become a powerful contaminant at 1200°C, leading to unexpected and undesirable chemical reactions on the material's surface.
Material Stability Limits
Every material has a temperature threshold. Pushing the temperature too high in search of speed can lead to internal structural problems.
These can include unwanted grain growth, which can make a metal brittle, or even physical distortion (warping) of the component.
Matching Temperature and Atmosphere to Your Goal
The optimal combination of temperature and atmosphere depends entirely on your intended outcome. There is no single "best" setting; there is only the right setting for a specific material and goal.
- If your primary focus is surface protection (e.g., bright annealing): Your goal is to use an inert atmosphere with the lowest possible contamination, as high temperatures will magnify the effect of any residual oxygen or moisture.
- If your primary focus is surface modification (e.g., carburizing): You must carefully control both temperature and atmosphere composition to drive the desired reaction at a predictable and controlled rate.
- If your primary focus is material purification (e.g., vacuum processing): High temperature is the tool used to increase the vapor pressure of contaminants, while the vacuum atmosphere acts as the transport mechanism to remove them from the system.
Ultimately, mastering this relationship between heat and environment is the key to transforming raw materials into high-performance components with precision and repeatability.
Summary Table:
| Temperature's Role | Furnace Atmosphere's Role | Combined Effect |
|---|---|---|
| Acts as an accelerator | Acts as a steering wheel | Determines final material properties |
| Provides activation energy for reactions | Dictates chemical environment at surface | Drives processes like carburizing or purification |
| Increases reaction rates (good & bad) | Protects (inert), Modifies (reactive), or Purifies (vacuum) | Requires precise control to avoid defects |
Master the precise interplay of temperature and atmosphere for your specific material processing goals. At KINTEK, we leverage our exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions. Our product line—including Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems—is engineered for superior control. Coupled with our strong deep customization capability, we can tailor a furnace system to precisely meet your unique experimental requirements, ensuring you achieve the perfect heat-environment balance for repeatable, high-quality results. Contact our experts today to discuss your application!
Visual Guide
Related Products
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- What are the two main types of atmosphere furnaces and their characteristics? Choose the Right Furnace for Your Lab
- What industries commonly use inert atmosphere heat treating? Key Applications in Military, Automotive, and More
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality



















