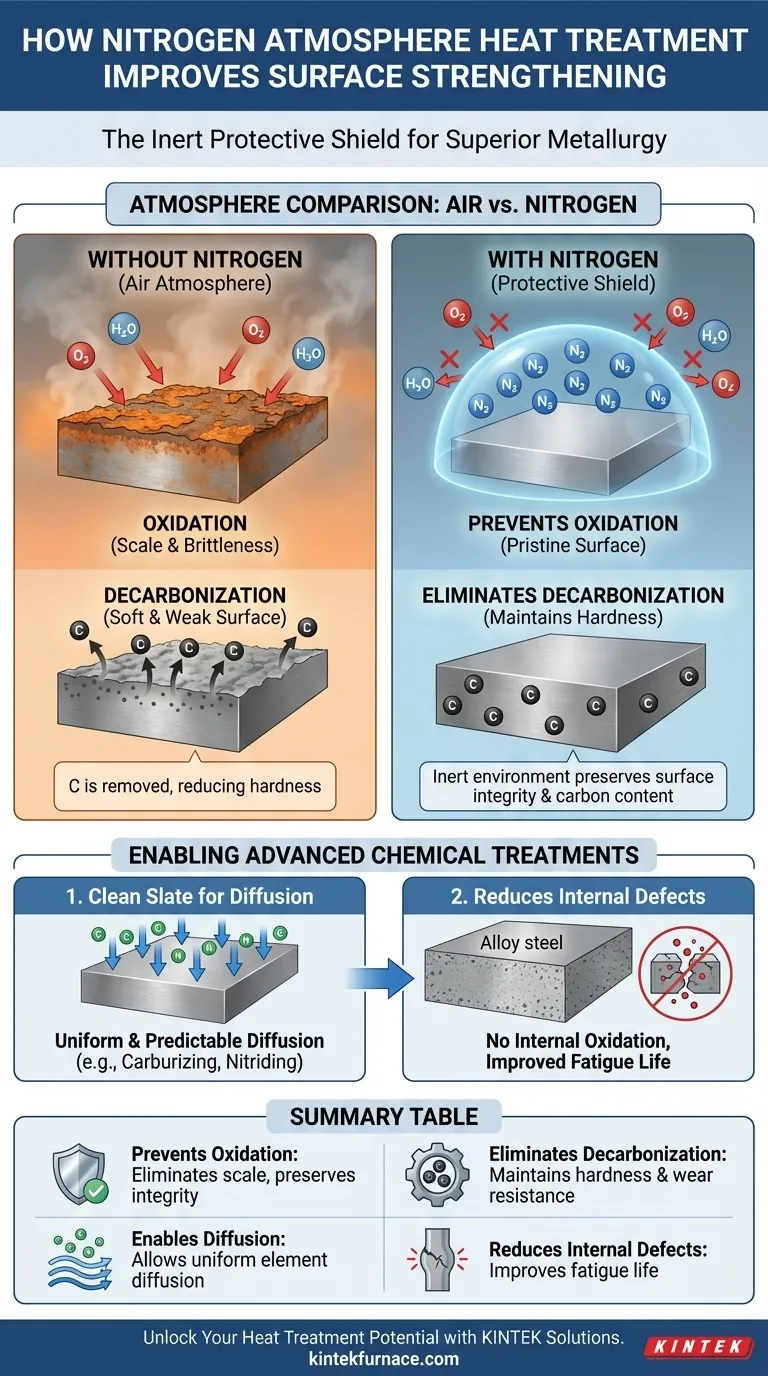
At its core, nitrogen atmosphere heat treatment improves surface strengthening by creating a chemically inert environment. This protective shield prevents the harmful reactions, such as oxidation and decarbonization, that would otherwise occur at high temperatures in the presence of air, allowing the intended strengthening mechanisms to work with maximum effectiveness.
The crucial insight is that in this context, nitrogen is not an active strengthening agent. Instead, it acts as a perfect, neutral bodyguard for the material's surface, preventing weakening defects and ensuring the success of the actual heat treatment process.

The Role of Nitrogen as a Protective Shield
To understand the benefit, we must first consider what happens during heat treatment in a normal air atmosphere. The high temperatures required for processes like hardening or annealing accelerate chemical reactions between the metal's surface and the surrounding air.
Preventing Oxidation
At elevated temperatures, oxygen readily reacts with the iron in steel to form a layer of iron oxide, commonly known as scale. This scale is brittle, flaky, and detrimental to the component's final properties.
A nitrogen atmosphere displaces the oxygen in the furnace, creating an environment where oxidation simply cannot occur. This preserves the pristine metallic surface of the part, eliminating the need for costly and potentially damaging post-treatment cleaning operations like sandblasting or acid pickling.
Eliminating Decarbonization
Decarbonization is another damaging reaction where oxygen or water vapor reacts with the carbon within the steel's surface layer, pulling it out of the material. Since carbon is the primary element responsible for the hardness of steel, its loss results in a soft, weak surface.
By providing an inert environment, a nitrogen atmosphere prevents these reactions, ensuring the carbon content—and therefore the potential hardness and wear resistance—of the surface remains exactly as designed.
Enabling Superior Chemical Heat Treatments
Many advanced strengthening techniques involve diffusing other elements into the steel's surface. A nitrogen atmosphere is not just protective; it is a fundamental prerequisite for the quality and reliability of these processes.
A Clean Slate for Diffusion
Processes like carburizing (adding carbon) or nitriding (adding nitrogen) rely on the diffusion of elements into the steel's surface. If an oxide layer is present, it acts as a barrier, blocking or hindering this diffusion.
This leads to uneven case depths and inconsistent hardness. A nitrogen-based atmosphere ensures the surface is perfectly clean, allowing for uniform and predictable diffusion, which is critical for high-performance components like gears and bearings.
Reducing Internal Defects
For alloy steels containing elements like chromium, manganese, or silicon, oxygen can cause an even more insidious problem: internal oxidation. Oxygen atoms can diffuse a short distance into the material and form microscopic oxide particles beneath the surface.
These internal oxides act as stress concentration points, severely reducing the material's fatigue life. A pure nitrogen atmosphere eliminates the source of oxygen, thereby preventing the formation of these strength-robbing internal defects.
Understanding the Trade-offs
While nitrogen-based atmospheres offer superior results, it's important to understand the context and why other methods exist.
Inert vs. Active Atmospheres
The focus here is on nitrogen as an inert carrier gas. Its job is to do nothing. This is distinct from processes like gas nitriding, where the atmosphere (often a mix of nitrogen and ammonia) is intentionally designed to be active and donate nitrogen atoms to the surface to form hard nitride compounds.
The reference to avoiding "nitrogen embrittlement" is key. Using pure, dry nitrogen as a shield prevents unwanted reactions. Improperly controlled atmospheres can inadvertently add too much nitrogen, leading to brittleness.
Comparison to Older Methods
The references note that older methods like steam treatment or controlled oxidation only increase tool life by 30-50%. This is because these processes create a thin, hard-but-brittle oxide layer (like black oxide) on the surface.
This oxide provides some wear and corrosion resistance but is fundamentally inferior to a surface strengthened through defect-free hardening or carburizing. Nitrogen atmospheres enable the latter, more robust strengthening mechanisms, yielding far superior performance and reliability.
Making the Right Choice for Your Goal
Selecting the correct furnace atmosphere is not just a procedural detail; it is foundational to achieving the desired metallurgical properties of the final component.
- If your primary focus is maximum surface hardness and fatigue strength: A nitrogen-based atmosphere is essential to prevent defects and ensure the success of hardening or chemical treatments.
- If your primary focus is low-cost corrosion and wear resistance for non-critical parts: An older, controlled oxidation process like steam bluing may be a sufficient and more economical choice.
- If your goal is to intentionally add nitrogen to the surface (nitriding): You will need a specific, active atmosphere containing a source of dissociated nitrogen, which is a different process from using nitrogen as a protective shield.
Ultimately, using a nitrogen atmosphere is a decision to control the process environment precisely, preventing random, detrimental reactions and guaranteeing the intended metallurgical transformation is achieved.
Summary Table:
| Key Aspect | Role in Surface Strengthening |
|---|---|
| Prevents Oxidation | Eliminates scale formation, preserving surface integrity |
| Eliminates Decarbonization | Maintains carbon content for hardness and wear resistance |
| Enables Diffusion | Allows uniform element diffusion in processes like carburizing |
| Reduces Internal Defects | Prevents internal oxidation, improving fatigue life |
Unlock the full potential of your heat treatment processes with KINTEK! Leveraging exceptional R&D and in-house manufacturing, we provide advanced high-temperature furnace solutions tailored to your needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all supported by deep customization capabilities to meet your unique experimental requirements. Whether you're aiming for maximum surface hardness, fatigue strength, or precise metallurgical transformations, KINTEK ensures reliable, defect-free results. Contact us today to discuss how our solutions can enhance your lab's efficiency and performance!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality



















