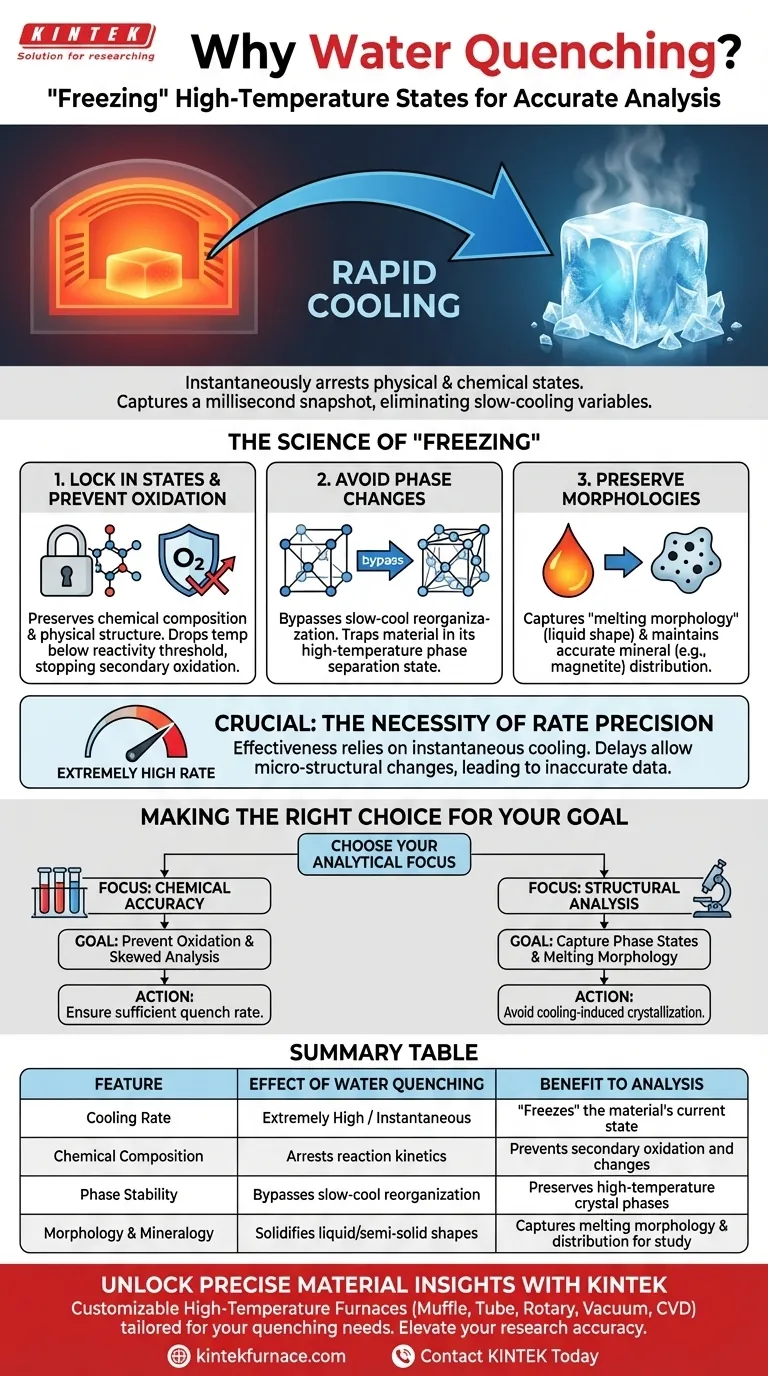
Water quenching is primarily employed to achieve an extremely high cooling rate that instantaneously "freezes" the material. By rapidly dropping the temperature, you effectively arrest the physical and chemical state of the reaction products, preventing them from evolving further as they leave the high-temperature environment.
The core purpose of water quenching is to capture an accurate snapshot of the material's high-temperature properties. It eliminates the variables introduced by slow cooling, ensuring that the recovered samples truly represent the conditions present during the millisecond-scale reaction.

The Science of "Freezing" High-Temperature States
Locking in Physical and Chemical States
At high temperatures, materials exist in dynamic states that often change rapidly.
Water quenching utilizes the high heat capacity of water to remove thermal energy almost instantly.
This process "freezes" the particles, preserving their chemical composition and physical structure exactly as they existed at the moment of the reaction.
Preventing Secondary Oxidation
When materials cool slowly in the presence of air or other gases, they are susceptible to secondary oxidation.
This means the surface or internal structure reacts with oxygen as the temperature decreases, creating oxides that were not present during the main reaction.
Quenching prevents this by dropping the temperature below the reactivity threshold before oxidation can occur.
Avoiding Phase Changes
Materials often undergo phase changes (changes in crystal structure or state) as they transition from high to low temperatures.
Slow cooling allows the material to reorganize into low-temperature stable phases.
Rapid quenching bypasses this reorganization, trapping the material in its high-temperature phase separation state.
Preserving Specific Morphologies
Capturing Melting Morphology
To understand how a material behaved inside the furnace, you must see its liquid or semi-solid shape.
Quenching solidifies the material so quickly that the "melting morphology"—the shape it took while molten—is preserved for analysis.
Magnetite Distribution
For processes involving iron ores or similar materials, the distribution of specific minerals like magnetite is critical.
Quenching ensures that the distribution pattern observed in the lab matches the distribution during the actual reaction.
Understanding the Trade-offs
The Necessity of Rate Precision
The effectiveness of this method relies entirely on the cooling rate being "extremely high."
If the quenching mechanism is delayed or the volume of water is insufficient, the cooling rate drops.
Even a slight delay can allow micro-structural changes to occur, rendering the sample an inaccurate representation of the high-temperature state.
Making the Right Choice for Your Goal
To ensure you extract valuable data from your high-temperature process, consider what specific attributes you need to analyze.
- If your primary focus is Chemical Accuracy: Ensure the quench rate is sufficient to prevent secondary oxidation, which would skew compositional analysis.
- If your primary focus is Structural Analysis: Use quenching to capture the specific phase separation states and melting morphology without the interference of cooling-induced crystallization.
Water quenching is the definitive method for turning a dynamic, millisecond-scale reaction into a static, analyzable sample.
Summary Table:
| Feature | Effect of Water Quenching | Benefit to Analysis |
|---|---|---|
| Cooling Rate | Extremely High / Instantaneous | "Freezes" the material's current state |
| Chemical Composition | Arrests reaction kinetics | Prevents secondary oxidation and changes |
| Phase Stability | Bypasses slow-cool reorganization | Preserves high-temperature crystal phases |
| Morphology | Solidifies liquid/semi-solid shapes | Captures melting morphology for study |
| Mineralogy | Stops mineral migration | Maintains accurate magnetite distribution |
Unlock Precise Material Insights with KINTEK
To capture accurate data from dynamic, millisecond-scale reactions, you need equipment designed for precision. At KINTEK, we specialize in providing high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for researchers and industrial experts.
Our lab high-temperature furnaces are fully customizable to meet your unique quenching and thermal processing requirements, ensuring your material's physical and chemical states are preserved exactly as they exist at peak temperatures.
Ready to elevate your research accuracy? Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Motoo KAWASAKI, Hiromichi Takebe. Evaluation of Ignition and Combustion Reactions of CuFeS<sub>2</sub> and Silica Stone Less Than 100 ms in a Drop Furnace. DOI: 10.2473/journalofmmij.mmij-2024-010
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- Why is precise temperature control in an aging oven critical for ZK61 alloys? Master the 175°C Pre-aging Threshold
- Which type of furnace is better for specific applications? Choose the Right Furnace for Your Production Needs
- Why is a graphite furnace better than a flame in AAS? Unlock Trace-Level Detection for Your Lab
- How do surface oxidation systems improve the interface performance of graphitized fibers? Maximize Composite Strength
- How does Plasma Flash Sintering (PFS) equipment enable the stabilization of metastable phases? Defy Thermal Limits
- Why is temperature control precision critical for a sample heating furnace? Master Ti-V-Cr Alloy Oxidation Kinetics
- How does uniform heating benefit furnace applications? Achieve Superior Quality and Efficiency
- What critical environment does a high-temp furnace provide for H13 steel? Mastering Microstructural Homogenization



















