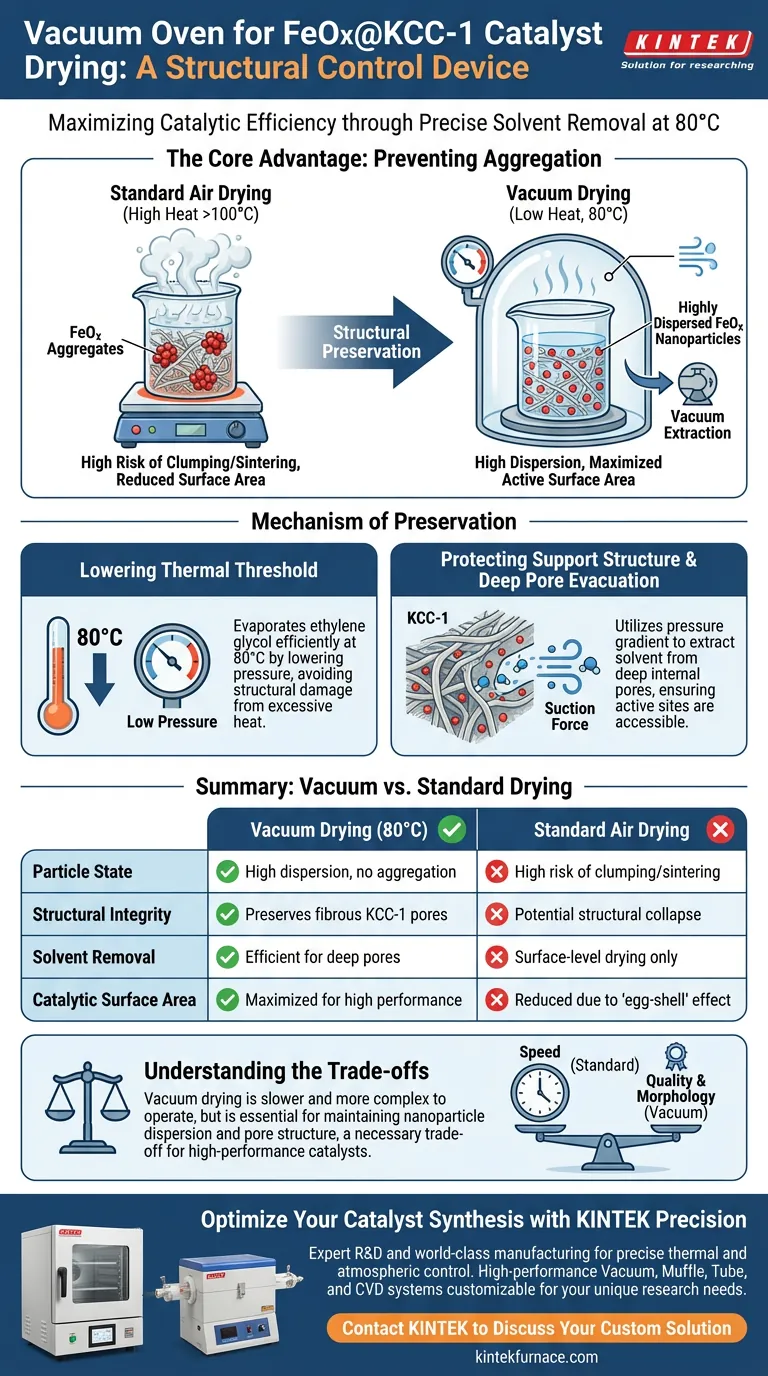
The primary reason for utilizing a vacuum oven in this process is to facilitate the complete evaporation of the ethylene glycol solvent at a controlled temperature of 80 °C. By lowering the atmospheric pressure, the oven enables the solvent to evaporate efficiently without requiring the excessive heat that would otherwise damage the catalyst's structure.
The core advantage of vacuum drying is the prevention of nanoparticle aggregation. By removing solvents at lower temperatures, the process locks the iron oxide (FeOx) particles in a highly dispersed state on the KCC-1 support, maximizing the active surface area available for catalysis.

The Mechanism of Particle Preservation
Lowering the Thermal Threshold
Standard drying methods often require high temperatures to overcome the boiling point of solvents like ethylene glycol.
Preventing Component Aggregation
The defining risk during the drying phase is aggregation, where active particles clump together. According to the primary technical data, standard air drying at higher temperatures induces the movement and clustering of active components. The vacuum environment mitigates this by allowing the material to dry effectively at 80 °C, ensuring the FeOx nanoparticles remain separate and distinct.
Protecting the Support Structure
Evacuation from Deep Pores
KCC-1 is a silica support known for its fibrous, high-surface-area structure. Vacuum drying utilizes a pressure gradient to extract solvent molecules from within the deep internal pores of the support. This ensures that the active sites inside the catalyst structure are cleared of solvent and accessible for reaction.
Maintaining High Dispersion
The efficiency of a catalyst is directly tied to how well the active material is spread out. By preventing the thermal sintering or clumping of particles, the vacuum process ensures a highly dispersed coating of FeOx. This high dispersion is critical for the final catalytic performance of the material.
Understanding the Trade-offs
Drying Speed vs. Structural Quality
While vacuum drying preserves morphology, it is not always the fastest method. As noted in comparative drying studies, vacuum drying rates can be lower than rapid convective drying methods. However, rapid methods often lead to uneven "egg-shell" distributions or structural collapse, making the slower vacuum process a necessary trade-off for quality.
Complexity of Operation
Vacuum drying requires maintaining a sealed system and operating a vacuum pump. This adds a layer of operational complexity compared to a standard laboratory oven. However, for nanomaterials where pore structure and particle size are paramount, this complexity is a required cost of production.
Making the Right Choice for Your Goal
To determine if this drying protocol aligns with your specific synthesis requirements, consider the following:
- If your primary focus is Catalytic Efficiency: Prioritize vacuum drying to ensure maximum dispersion of nanoparticles and the highest possible active surface area.
- If your primary focus is Process Speed: You might consider standard convective drying, but you must accept the high risk of particle aggregation and reduced performance.
Ultimately, the vacuum oven is not just a drying tool; it is a structural control device that ensures the microscopic integrity of your FeOx@KCC-1 catalyst.
Summary Table:
| Feature | Vacuum Drying (80 °C) | Standard Air Drying |
|---|---|---|
| Particle State | High dispersion, no aggregation | High risk of clumping/sintering |
| Structural Integrity | Preserves fibrous KCC-1 pores | Potential structural collapse |
| Solvent Removal | Efficient for deep pores | Surface-level drying only |
| Catalytic Surface Area | Maximized for high performance | Reduced due to 'egg-shell' effect |
| Mechanism | Pressure gradient evaporation | High-heat thermal evaporation |
Optimize Your Catalyst Synthesis with KINTEK Precision
Precise structural control is the difference between a failing catalyst and a high-performance breakthrough. At KINTEK, we understand that maintaining nanoparticle dispersion in materials like FeOx@KCC-1 requires exact thermal and atmospheric conditions.
Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Vacuum, Muffle, Tube, and CVD systems designed to protect your most delicate samples. Our lab high-temp furnaces are fully customizable to meet your unique research or production needs, ensuring your active sites remain accessible and your support structures intact.
Ready to elevate your lab's drying and heating capabilities? Contact KINTEK today to discuss your custom solution
Visual Guide
References
- Guobo Li, Honggen Peng. Unraveling FeOx Nanoparticles Confined on Fibrous Mesoporous Silica Catalyst Construction and CO Catalytic Oxidation Performance. DOI: 10.3390/catal14010063
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is vacuum brazing preferred in the aerospace industry? For Strong, Clean, and Reliable Joints
- What are the advantages of using a vacuum drying oven for PB2T-TEG-TiO2-X? Protect Sensitive Polymers & Prevent Oxidation
- What are the advantages of using a vacuum furnace? Achieve Superior Heat Treatment with Precision Control
- What is the temperature of a vacuum furnace brazing? Optimize Your Joint Strength and Cleanliness
- How do customized vacuum furnaces meet specific process requirements? Tailor Your Heat Treatment for Maximum Efficiency
- What are the five main components of a vacuum melting furnace? Essential for High-Purity Metal Production
- What are the advantages of vacuum brazed connections? Achieve Strong, Clean, and Reliable Joints
- Why is vacuum-pressure treatment equipment required for deep wood impregnation? Unlock Ultimate Material Durability



















