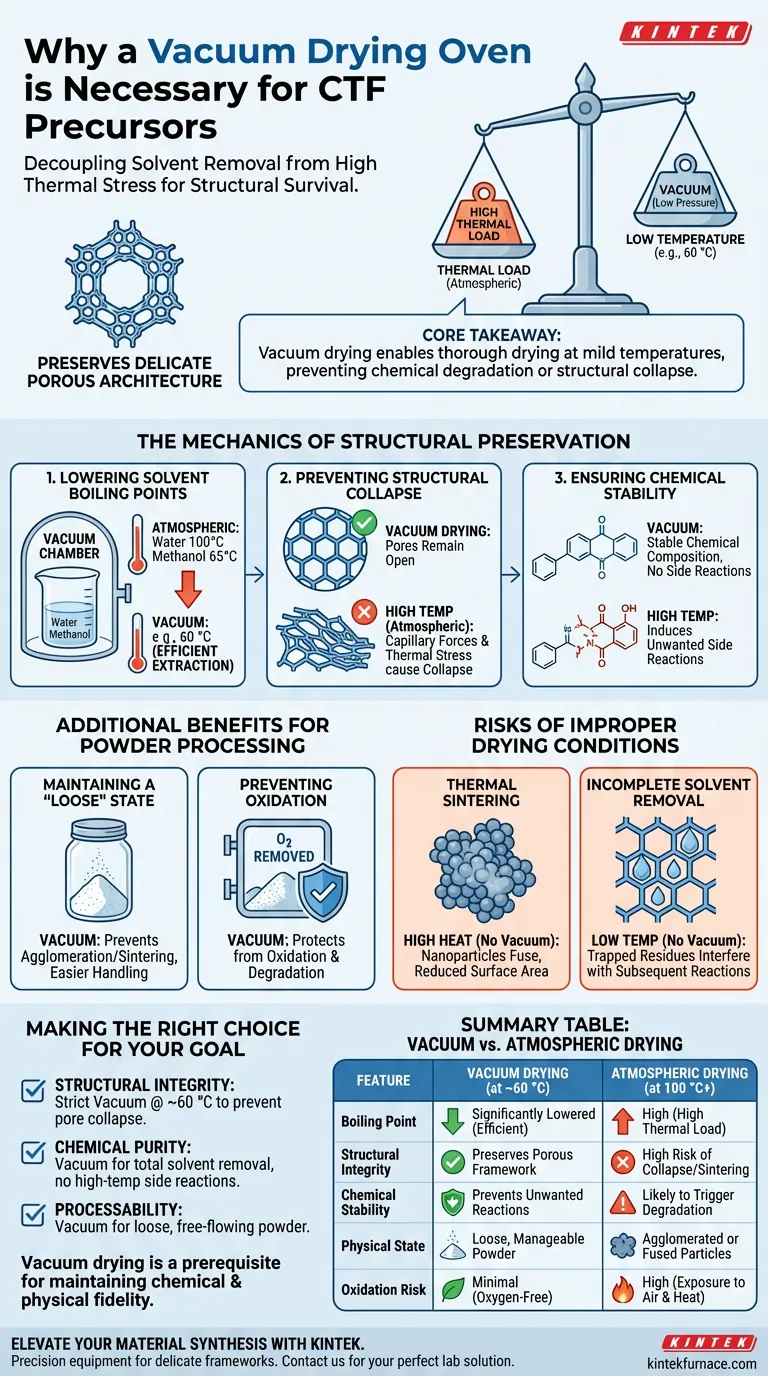
Vacuum drying is a critical process control step used to ensure the structural survival of the material. It is strictly necessary during the preparation of covalent triazine framework (CTF) precursors to effectively remove residual solvents, such as methanol and water, at controlled, low temperatures (typically around 60 °C).
By operating in a low-pressure environment, the oven reduces the boiling points of these solvents, allowing them to evaporate without subjecting the precursor to the high heat that would otherwise be required at atmospheric pressure.
Core Takeaway: The vacuum drying oven decouples solvent removal from high thermal stress. By lowering the boiling point of residual liquids, it enables thorough drying at mild temperatures. This preserves the delicate porous architecture of the covalent triazine framework and prevents chemical degradation or structural collapse.
The Mechanics of Structural Preservation
Lowering Solvent Boiling Points
The fundamental function of the vacuum oven is to manipulate the physical properties of the solvents trapped within the material.
Under standard atmospheric pressure, removing solvents like water or methanol requires heating them to 100 °C or 65 °C, respectively.
By creating a vacuum, the boiling points of these liquids drop significantly. This allows the solvents to be extracted efficiently at a much lower operational temperature, such as 60 °C, reducing the thermal load on the precursor.
Preventing Structural Collapse
Covalent triazine frameworks rely on a specific, porous architecture to function correctly.
If exposed to high temperatures during drying, the capillary forces and thermal stress can cause this polymer structure to collapse.
Vacuum drying mitigates this risk. It ensures that the pores remain open and the framework retains its intended geometry, which is essential for the material's final application.
Ensuring Chemical Stability
High temperatures do not just affect physical structure; they can trigger unwanted chemical changes.
Drying at atmospheric pressure often requires heat levels that induce unnecessary side reactions within the precursor mix.
The vacuum environment prevents these reactions, ensuring the chemical composition of the precursors remains stable and accurate relative to the synthesis design.
Additional Benefits for Powder Processing
Maintaining a "Loose" Physical State
Beyond chemical stability, the physical state of the dried powder is crucial for handling.
Vacuum drying prevents the material from undergoing severe agglomeration or sintering, which often occurs when wet materials are heated in air.
This ensures the precursor powder remains in a loose, manageable state, facilitating easier grinding or processing in subsequent steps.
Preventing Oxidation
While the primary goal is solvent removal, the vacuum environment inherently removes oxygen from the drying chamber.
This protects the precursor from oxidation, which can degrade the material before it even reaches the final processing stage.
Risks of Improper Drying Conditions
Thermal Sintering
Without the reduced pressure of a vacuum, achieving the same level of dryness requires higher temperatures.
This excess heat often leads to thermal sintering, where nanoparticles fuse together, drastically reducing the specific surface area of the material.
Incomplete Solvent Removal
Attempting to dry at low temperatures without a vacuum often results in trapped residues.
Residual solvents left in the pores can interfere with subsequent reactions or pyrolysis processes, leading to impurities or unpredictable material behaviors.
Making the Right Choice for Your Goal
To ensure the highest quality CTF precursors, tailor your drying protocol to your specific priorities:
- If your primary focus is Structural Integrity: strict adherence to vacuum drying at ~60 °C is required to prevent pore collapse.
- If your primary focus is Chemical Purity: utilize the vacuum to ensure total solvent removal without triggering high-temp side reactions.
- If your primary focus is Processability: rely on vacuum drying to keep the resulting powder loose and free of hard agglomerates.
Vacuum drying is not merely a method of speeding up evaporation; it is a prerequisite for maintaining the chemical and physical fidelity of your precursor.
Summary Table:
| Feature | Vacuum Drying (at ~60 °C) | Atmospheric Drying (at 100 °C+) |
|---|---|---|
| Boiling Point | Significantly lowered for efficient removal | High (requires high thermal load) |
| Structural Integrity | Preserves porous framework geometry | High risk of pore collapse/sintering |
| Chemical Stability | Prevents unwanted side reactions | Likely to trigger chemical degradation |
| Physical State | Loose, manageable powder | Agglomerated or fused particles |
| Oxidation Risk | Minimal (oxygen-free environment) | High (exposure to air and heat) |
Elevate Your Material Synthesis with KINTEK
Precision is paramount when preparing delicate covalent triazine frameworks. Backed by expert R&D and manufacturing, KINTEK offers advanced vacuum drying systems, muffle furnaces, and CVD systems designed to protect your material’s architecture. Whether you need customizable high-temp solutions or precise low-pressure control, our equipment ensures your precursors remain stable and pure.
Ready to optimize your drying process? Contact our experts today to find the perfect lab solution for your unique research needs!
Visual Guide
References
- Xin Pan, Qianqian Zhu. Nitrogen-Doped Porous Carbon Derived from Covalent Triazine Framework for Catalytic Oxidation of Benzyl Alcohol. DOI: 10.3390/nano14090744
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is the mechanism of a vacuum sintering furnace for AlCoCrFeNi2.1 + Y2O3? Optimize Your High-Entropy Alloy Processing
- What operational advantages does a vacuum heat treatment furnace offer? Achieve Superior Metallurgical Quality and Precision
- How do continuous vacuum furnaces enhance production efficiency in large-scale metal processing? Boost Throughput and Quality
- How is chamber customization beneficial in vacuum furnaces? Boost Purity, Efficiency, and Performance
- How are vacuum furnaces used in lithium battery materials preparation? Achieve High Purity and Performance
- What is the Bell Jar Furnace designed for? Achieve Ultra-Clean Processing for Sensitive Components
- What is the heat treatment in a vacuum furnace? Achieve Superior Metallurgical Properties
- What are the benefits of using graphite heating elements in vacuum furnaces? Achieve Extreme Heat and Durability



















