Precise control over the chemical environment is non-negotiable. A tube reduction furnace with reducing gas flow is necessary because it provides the only reliable method to chemically strip oxygen from high-valence uranium oxides without damaging the material. This system allows for the conversion of triuranium octoxide into uranium dioxide while simultaneously protecting the newly formed sub-stoichiometric powder from re-absorbing oxygen during the critical cooling phase.
The tube reduction furnace functions as a calibrated chemical reactor, using thermal stability and gas flow to precisely lower the oxidation state of uranium powder and lock it in that state during cooling.

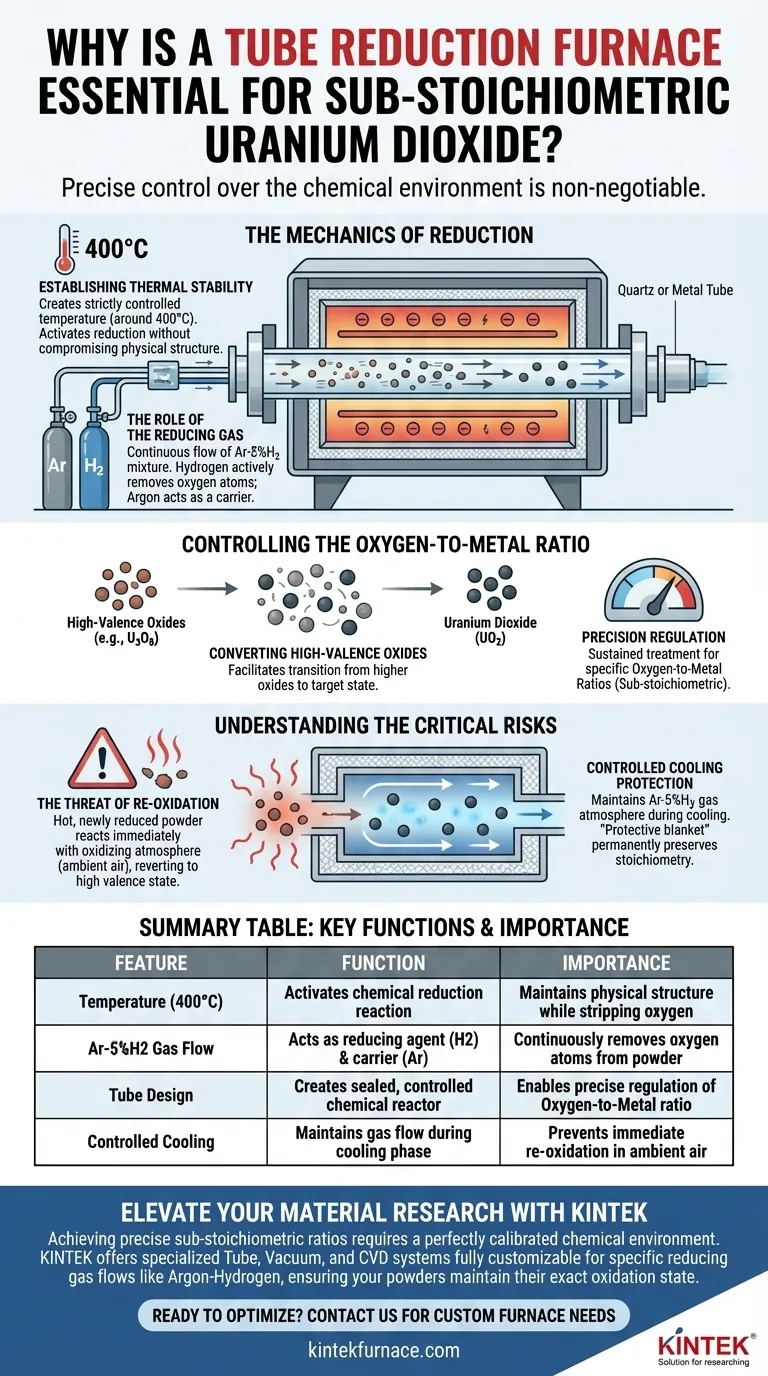
The Mechanics of Reduction
Establishing Thermal Stability
The primary function of the furnace is to create a strictly controlled temperature environment.
For this specific reduction process, the furnace maintains a temperature around 400°C. This specific heat level provides the necessary energy to activate the reduction reaction without compromising the physical structure of the powder.
The Role of the Reducing Gas
Heat alone is insufficient to change the chemical composition; a chemical agent is required.
The tube design supports the continuous flow of a reducing gas mixture, typically Argon-5% Hydrogen (Ar-5%H2). The hydrogen component actively reacts with the oxygen atoms in the uranium powder to remove them, while the argon serves as a stable carrier gas.
Controlling the Oxygen-to-Metal Ratio
Converting High-Valence Oxides
The starting material often consists of triuranium octoxide, which exists in a high-valence state.
To prepare useful uranium dioxide powders, this high-valence material must be chemically reduced. The furnace environment facilitates the transition from higher oxides down to the target uranium dioxide state.
Precision Regulation
Creating "sub-stoichiometric" powder requires hitting a very specific target.
Sustained treatment within the furnace allows for the precise regulation of the oxidation state. By controlling the duration of exposure and gas flow, operators can achieve specific oxygen-to-metal ratios rather than a generic composition.
Understanding the Critical Risks
The Threat of Re-oxidation
The most vulnerable moment in powder preparation occurs immediately after the heating cycle completes.
If the newly reduced powder is exposed to an oxidizing atmosphere (like ambient air) while still hot, it will react immediately. This re-oxidation reverts the material back to a higher valence state, effectively ruining the batch.
Controlled Cooling Protection
The tube furnace design mitigates this risk by maintaining the gas atmosphere during the cooling process.
The flow of the reducing gas mixture continues until the material reaches a safe temperature. This "protective blanket" ensures the specific stoichiometry achieved during heating is preserved permanently.
Making the Right Choice for Your Goal
To ensure successful powder preparation, align your process parameters with your specific objectives:
- If your primary focus is Precise Stoichiometry: Ensure the furnace temperature is strictly maintained at 400°C to facilitate a consistent reduction rate.
- If your primary focus is Material Purity: Verify that the flow of Ar-5%H2 is sustained throughout the entire cooling cycle to prevent surface re-oxidation.
Success in this process depends on viewing the furnace not just as a heater, but as a sealed instrument for chemical precision.
Summary Table:
| Feature | Function in Uranium Reduction | Importance for Sub-Stoichiometry |
|---|---|---|
| Temperature (400°C) | Activates the chemical reduction reaction | Maintains physical structure while stripping oxygen |
| Ar-5%H2 Gas Flow | Acts as a reducing agent (Hydrogen) and carrier (Argon) | Continuously removes oxygen atoms from the powder |
| Tube Design | Creates a sealed, controlled chemical reactor | Enables precise regulation of the oxygen-to-metal ratio |
| Controlled Cooling | Maintains gas flow during the cooling phase | Prevents immediate re-oxidation in ambient air |
Elevate Your Material Research with KINTEK
Achieving precise sub-stoichiometric ratios requires more than just heat; it requires a perfectly calibrated chemical environment. Backed by expert R&D and manufacturing, KINTEK offers specialized Tube, Vacuum, and CVD systems designed for the most demanding lab requirements. Our high-temperature furnaces are fully customizable to handle specific reducing gas flows like Argon-Hydrogen, ensuring your powders maintain their exact oxidation state from heating through cooling.
Ready to optimize your powder preparation? Contact us today to discuss your custom furnace needs!
Visual Guide
References
- Lee Shelly, Shmuel Hayun. Unveiling the factors determining water adsorption on CeO <sub>2</sub> , ThO <sub>2</sub> , UO <sub>2</sub> and their solid solutions. DOI: 10.1007/s12598-025-03393-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
People Also Ask
- What are the advantages of using a laboratory tube furnace for BCZT ceramics? Boost Piezoelectric d33 by up to 41%
- Why does a tube sintering furnace require precise control for (RuIr)O2/C catalysts? Optimize Catalyst Performance
- Why is a stable nitrogen flow required in a tube furnace for hydrochar carbonization? Ensure High-Carbon Purity
- How is a tube high-temperature furnace utilized in the preparation of NiSA-O/Mo2C catalysts? Expert Synthesis Guide
- What role does a Vertical Tube Furnace play in ferronickel reduction smelting? Expert Process Simulation
- How do laboratory-scale Tube Furnaces facilitate coal gasification? Precise Simulation for Industrial Success
- How does a high-temperature tube furnace ensure magnesium alloy scaffold performance? Expert Sintering Guide
- Why is the space-saving design of a tube furnace advantageous? Unlock Efficiency in Your Lab



















