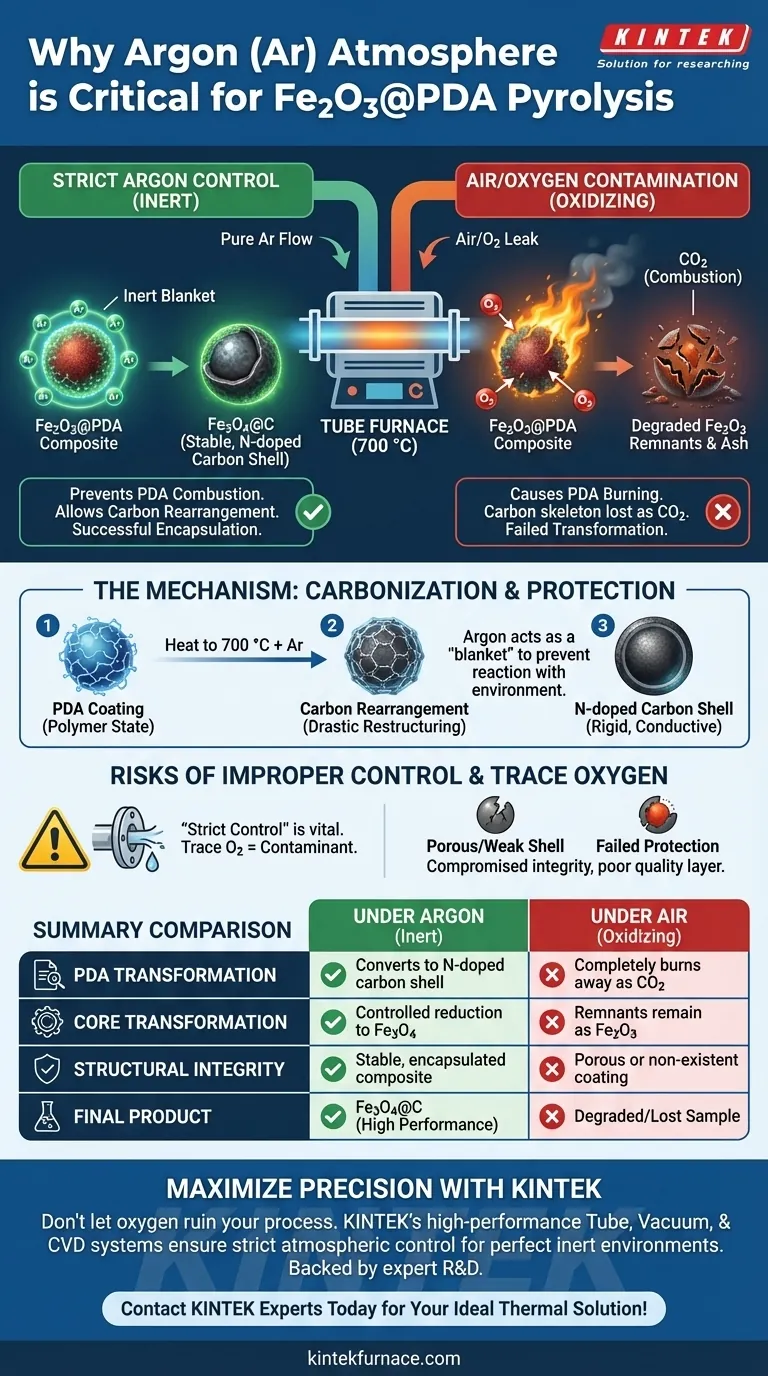
A strictly controlled argon (Ar) atmosphere is required to prevent the combustion of the polydopamine (PDA) layer during high-temperature processing. Without this inert environment, the oxygen present in the air would react with the carbon precursor at 700 °C, burning it away completely rather than converting it into a functional shell.
The primary role of the argon atmosphere is to exclude oxygen, preventing the oxidative loss of the carbon skeleton. This allows the polydopamine coating to successfully transform into a nitrogen-doped carbon layer, encapsulating the core to form a stable Fe3O4@C structure.

The Mechanism of Carbonization
Transforming Polydopamine (PDA)
The process relies on heating the material to approximately 700 °C. At this temperature, the PDA coating undergoes a drastic chemical restructuring. It transitions from a polymer state into a rigid, nitrogen-doped carbon layer.
Preserving the Carbon Skeleton
This transformation is delicate. For the PDA to become a conductive carbon shell, the carbon atoms must rearrange themselves rather than react with the environment. Argon provides the necessary inert "blanket" that allows this rearrangement to occur undisturbed.
The Critical Role of Oxygen Exclusion
Preventing Oxidative Loss
If oxygen enters the tube furnace, the high temperatures will trigger immediate oxidation. Instead of forming a solid shell, the carbon atoms will bind with oxygen to form carbon dioxide (CO2). This results in the total loss of the coating materials and intermediate products.
Facilitating Phase Transformation
The goal is to create an Fe3O4@C structure from the original Fe2O3@PDA composite. The inert atmosphere supports this by allowing the thermal reduction of the iron oxide core while simultaneously creating the protective carbon encapsulation.
Risks of Improper Atmosphere Control
The "Strict Control" Requirement
Simply introducing argon is not enough; the environment must be strictly controlled. Any leakage or residual air in the tube furnace acts as a contaminant.
Compromised Structural Integrity
Even trace amounts of oxygen can degrade the quality of the nitrogen-doped carbon layer. This leads to a porous, weak, or non-existent shell that fails to protect the metal oxide framework.
Making the Right Choice for Your Goal
To ensure the successful synthesis of Fe3O4@C composites, consider the following operational priorities:
- If your primary focus is maximizing shell thickness: Ensure the tube furnace is thoroughly purged before heating to remove all residual oxygen that could consume the carbon precursor.
- If your primary focus is phase purity (Fe3O4 formation): Maintain a steady, positive pressure of argon throughout the 700 °C hold time to prevent re-oxidation of the iron core.
Strict atmospheric control is the difference between burning your sample and engineering a high-performance functional material.
Summary Table:
| Feature | Under Argon (Inert) | Under Air (Oxidizing) |
|---|---|---|
| PDA Transformation | Converts to N-doped carbon shell | Completely burns away as CO2 |
| Core Transformation | Controlled reduction to Fe3O4 | Remnants likely remain as Fe2O3 |
| Structural Integrity | Stable, encapsulated composite | Porous or non-existent coating |
| Final Product | Fe3O4@C (High performance) | Degraded/Lost sample |
Maximize Your Material Synthesis Precision with KINTEK
Don't let oxygen contamination ruin your complex carbonization processes. KINTEK's high-performance Tube, Vacuum, and CVD systems are engineered for the strict atmospheric control required for advanced material research like Fe2O3@PDA pyrolysis. Backed by expert R&D and manufacturing, our customizable laboratory furnaces ensure a perfectly inert environment to preserve your carbon skeletons and achieve phase purity every time.
Ready to elevate your lab's results? Contact KINTEK experts today to find the ideal thermal solution for your unique needs!
Visual Guide
References
- Yan Yan, Jie Zeng. General synthesis of neighboring dual-atomic sites with a specific pre-designed distance via an interfacial-fixing strategy. DOI: 10.1038/s41467-024-55630-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What types of industries commonly use box-type atmosphere furnaces? Essential for Metallurgy, Electronics, and More
- What are the key features of a retort furnace? Unlock Precise Atmospheric Control for Advanced Processes
- What is the purpose of using flowing nitrogen during annealing? Protect Music Wire Integrity
- What physical conditions must a high-temp reduction furnace provide for Ni exsolution? Master Your Material Synthesis
- What is the role of an atmosphere sintering furnace in the MLM process? Master CNT/Cu Composite Preparation
- What is the function of a high-pressure Argon atmosphere? Master Complex Alloy Purity with Precision Melting
- What atmospheric environment is required for MIM catalytic debinding? Achieve 99.999% Purity for Perfect Metal Parts
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment



















