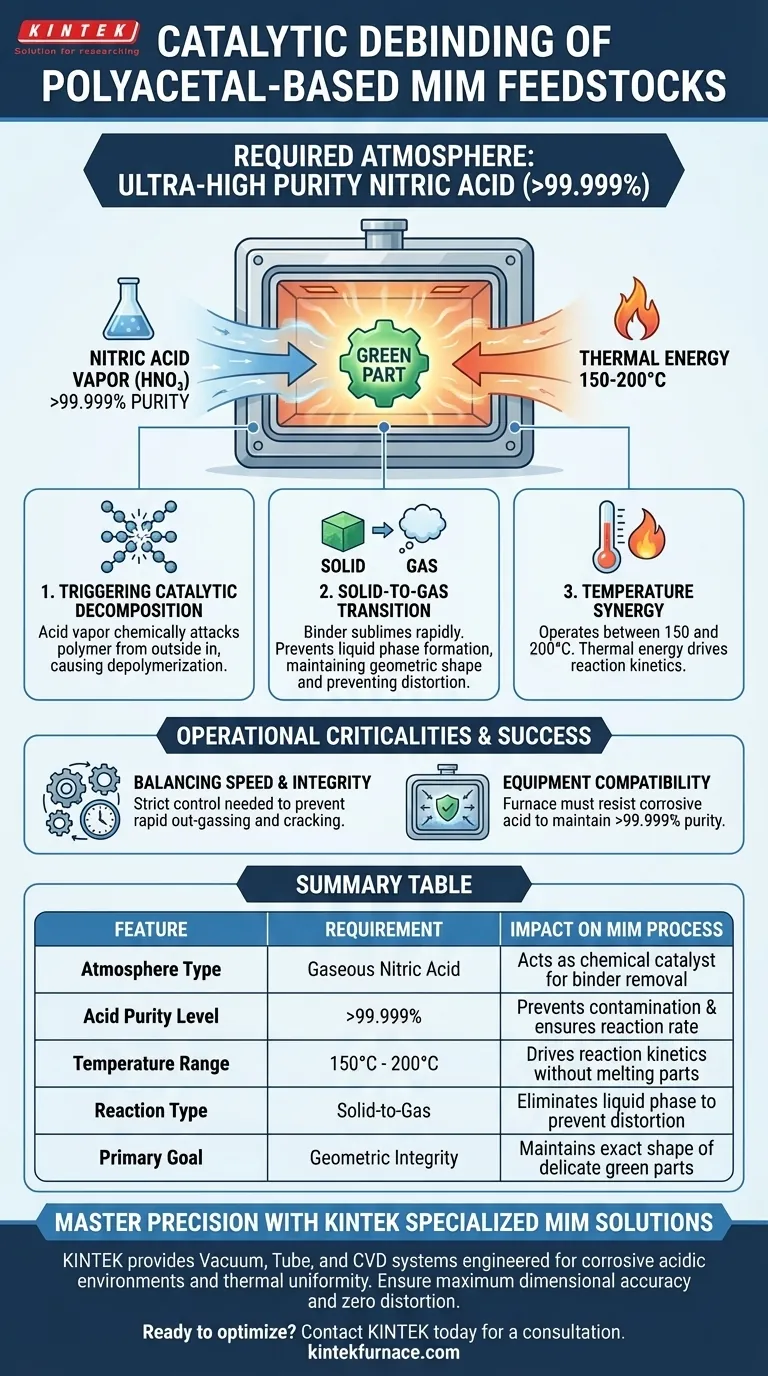
The catalytic debinding of polyacetal-based MIM feedstocks demands a strictly controlled, ultra-high purity nitric acid atmosphere. Specifically, the process requires nitric acid with a purity level exceeding 99.999%. This highly acidic environment acts as a chemical catalyst, enabling the rapid decomposition of the binder components without melting them.
Core Insight The success of this process relies on the synergy between strong acidity and heat. By introducing >99.999% purity nitric acid into the debinding oven, you trigger a direct solid-to-gas transition of the binder, which is the only way to remove the polymer while ensuring the metal component retains its exact geometric shape.
The Role of the Acidic Atmosphere
Triggering Catalytic Decomposition
The nitric acid vapor does not simply wash away the binder; it chemically attacks the polymer chains. This catalytic reaction causes the polyacetal binder to depolymerize from the outside of the part inward.
The Necessity of Ultra-High Purity
The standard requires nitric acid exceeding 99.999% purity. Using lower-grade acid can introduce contaminants that interfere with the reaction rate or leave unwanted residues in the porous metal structure.
Process Conditions and Mechanism
Temperature Synergy
While the acid provides the chemical trigger, thermal energy drives the kinetics of the reaction. The process operates effectively at temperatures between 150 and 200 °C.
Preventing Distortion
Because the reaction occurs in this specific temperature range under acidic conditions, the binder decomposes rapidly directly into a gas. This prevents the formation of a liquid phase, ensuring the "green part" does not slump or lose its geometric shape during binder removal.
Operational Criticalities
Balancing Reaction Speed and Integrity
The combination of high-purity acid and temperatures up to 200 °C is aggressive. While this facilitates speed, strict control is required to prevent rapid out-gassing that could crack delicate features.
Equipment Compatibility
Creating an atmosphere of highly concentrated, hot nitric acid requires specialized equipment. The furnace chamber and gas flow systems must be chemically resistant to this specific corrosive environment to maintain the 99.999% purity level throughout the cycle.
Ensuring Process Success
If your primary focus is Dimensional Accuracy:
- Maintain the nitric acid purity strictly above 99.999% to ensure the reaction remains purely catalytic, preventing partial melting or distortion.
If your primary focus is Process Efficiency:
- Optimize your thermal profile within the 150-200 °C window to maximize the decomposition rate provided by the acidic atmosphere.
The integrity of your final metal part is directly correlated to the purity of the nitric acid atmosphere used during this critical intermediate step.
Summary Table:
| Feature | Requirement | Impact on MIM Process |
|---|---|---|
| Atmosphere Type | Gaseous Nitric Acid | Acts as chemical catalyst for binder removal |
| Acid Purity Level | >99.999% | Prevents contamination & ensures reaction rate |
| Temperature Range | 150°C - 200°C | Drives reaction kinetics without melting parts |
| Reaction Type | Solid-to-Gas | Eliminates liquid phase to prevent distortion |
| Primary Goal | Geometric Integrity | Maintains exact shape of delicate green parts |
Master Precision with KINTEK Specialized MIM Solutions
Achieving the strict 99.999% purity and temperature control required for catalytic debinding demands high-performance equipment. KINTEK provides industry-leading R&D and manufacturing of specialized Vacuum, Tube, and CVD systems, specifically engineered to withstand corrosive acidic environments while maintaining thermal uniformity.
Whether you need customizable lab furnaces or high-temp industrial systems, our expert-designed solutions ensure your metal injection molding process delivers maximum dimensional accuracy and zero distortion.
Ready to optimize your debinding process? Contact KINTEK today for a consultation and let our experts help you select the perfect system for your unique manufacturing needs.
Visual Guide
References
- Jorge Luis Braz Medeiros, Luciano Volcanoglo Biehl. Effect of Sintering Atmosphere Control on the Surface Engineering of Catamold Steels Produced by MIM: A Review. DOI: 10.3390/surfaces9010007
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
People Also Ask
- How does a horizontal box furnace facilitate atmosphere control in the synthesis of Ni-TiON catalysts?
- What is a brazing furnace? Achieve Superior, Oxidation-Free Metal Joining
- How is a controlled atmosphere furnace used in material research? Achieve Precise Material Synthesis and Heat Treatment
- How does a high-temperature electric furnace facilitate the sintering process of 3Y-TZP ceramics? Master Densification
- What are the main types of nitrogen-based furnace atmospheres? Optimize Your Heat Treatment Process
- How does a retort furnace differ from other types of furnaces? Unlock Precision in Controlled Atmosphere Heating
- What are the cost considerations when using argon in furnaces? Balance Price vs. Material Integrity
- Why is a melting furnace with a constant argon flow required? Ensure Purity in Iodine-Bearing Glass Production



















