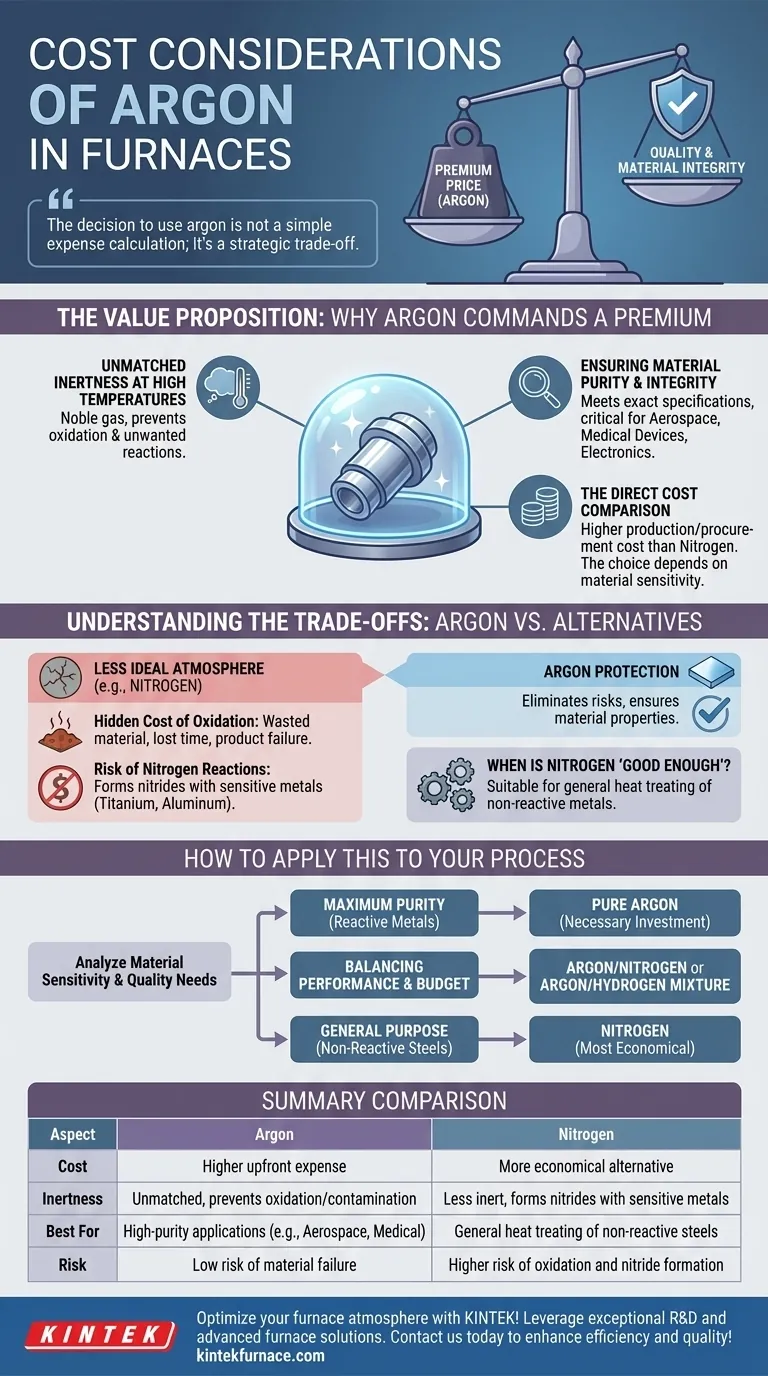
When evaluating furnace atmospheres, the primary cost consideration for argon is its premium price relative to alternatives like nitrogen. This higher upfront expense is a direct result of its unique chemical properties. However, for high-stakes applications where material integrity is non-negotiable, the cost of argon is often viewed as an investment in quality and a safeguard against the much higher costs associated with component failure or contamination.
The decision to use argon is not a simple expense calculation; it's a strategic trade-off. You are balancing the higher direct cost of the gas against the significant, and often hidden, downstream costs of oxidation, material contamination, and product rejection.
The Value Proposition: Why Argon Commands a Premium
Argon's cost is directly tied to its superior performance as a protective atmosphere in high-temperature environments. Understanding its benefits clarifies why it is often the preferred, albeit more expensive, choice.
Unmatched Inertness at High Temperatures
Argon is a noble gas, meaning it is almost completely non-reactive with other elements, even under extreme heat. This inertness is its most valuable property in furnace applications.
It creates a pristine environment that prevents unwanted chemical reactions, chiefly oxidation, from occurring on the surface of the material being treated.
Ensuring Material Purity and Integrity
By preventing oxidation and other reactions, argon ensures the final product meets exact metallurgical and chemical specifications. This is critical in industries like aerospace, medical device manufacturing, and electronics.
For these applications, even microscopic levels of contamination can lead to catastrophic component failure, making the integrity provided by argon essential.
The Direct Cost Comparison
Objectively, argon is more expensive to produce and procure than nitrogen. For applications where the risks of reaction are low, nitrogen presents a more economical alternative. The choice, therefore, depends entirely on the sensitivity of the material being processed.
Understanding the Trade-offs: Argon vs. Alternatives
Choosing a furnace gas is a risk management decision. The lower cost of an alternative like nitrogen must be weighed against the potential for negative reactions with your specific materials.
The Hidden "Cost" of Oxidation
Using a less-than-ideal atmosphere can lead to oxidation, which degrades the material's surface, compromises its structural integrity, and can cause the entire batch to be scrapped.
The cost of wasted material, lost production time, and potential product failure in the field almost always exceeds the savings gained by using a cheaper gas.
The Risk of Nitrogen Reactions
While often considered inert, nitrogen can react with certain metals at high temperatures to form nitrides. This is a significant issue for materials like titanium, aluminum, and certain stainless steels.
This nitride formation can alter the material's properties in undesirable ways, a risk that is completely eliminated by using chemically inactive argon.
When Is Nitrogen "Good Enough"?
For general heat treating of common carbon steels and other non-reactive metals, nitrogen is a perfectly suitable and highly cost-effective solution. If your process does not involve materials sensitive to nitride formation, the added cost of argon provides no significant benefit.
How to Apply This to Your Process
Your decision should be guided by a clear analysis of your material's sensitivity and your product's quality requirements.
- If your primary focus is maximum material purity for reactive metals (e.g., titanium, specialty alloys): The cost of pure argon is a necessary investment to prevent catastrophic material failure and ensure product quality.
- If your primary focus is balancing performance and budget: An argon/nitrogen or argon/hydrogen gas mixture can provide enhanced protection over pure nitrogen at a lower cost than pure argon.
- If your primary focus is general-purpose heat treating of non-reactive steels: Nitrogen is the most economical and technically sound choice for your operation.
Ultimately, selecting the right furnace gas is a strategic decision that aligns your operational costs with your quality requirements.

Summary Table:
| Aspect | Argon | Nitrogen |
|---|---|---|
| Cost | Higher upfront expense | More economical alternative |
| Inertness | Unmatched, prevents oxidation and contamination | Less inert, can form nitrides with sensitive metals |
| Best For | High-purity applications (e.g., aerospace, medical devices) | General heat treating of non-reactive steels |
| Risk | Low risk of material failure | Higher risk of oxidation and nitride formation for sensitive materials |
Optimize your furnace atmosphere for superior results with KINTEK! Leveraging exceptional R&D and in-house manufacturing, we provide advanced high-temperature furnace solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capabilities ensure precise solutions for your unique experimental needs. Don't let gas costs compromise your material integrity—contact us today to discuss how we can enhance your process efficiency and product quality!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- How does a batch type controlled atmosphere furnace operate? Master Precision Heat Treatment for Superior Materials
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance



















