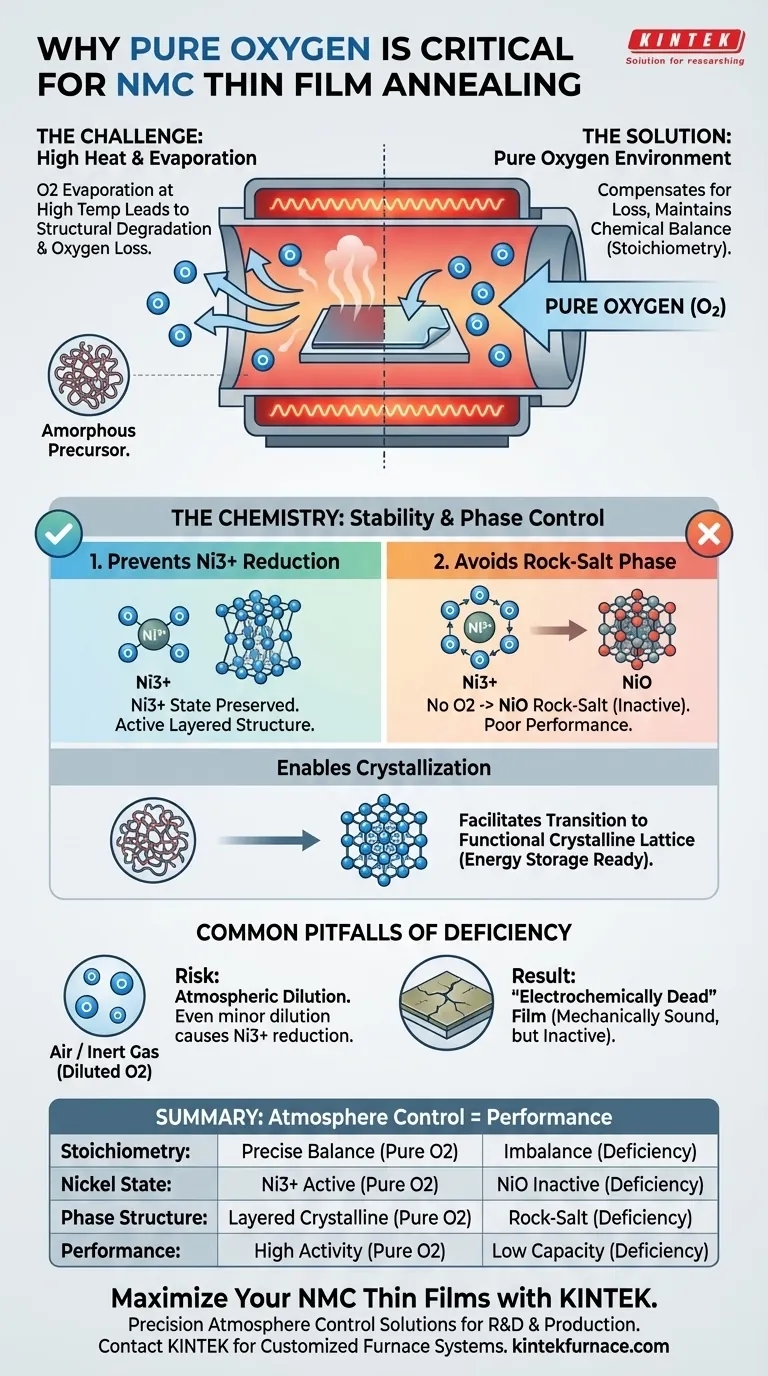
A pure oxygen environment is critical when annealing NMC thin films to strictly control the chemical composition of the material during high-temperature processing. Without this controlled atmosphere, the films lose oxygen due to evaporation, leading to irreversible structural degradation and poor electrochemical performance.
High-temperature annealing in pure oxygen compensates for evaporative losses, preventing the formation of inactive rock-salt phases and ensuring the film transitions correctly from an amorphous state to a crystalline, electrochemically active structure.

The Chemistry of Stability at High Heat
Counteracting Material Evaporation
Annealing processes typically require high temperatures to set the material structure. However, these elevated temperatures cause evaporation, leading to a significant loss of oxygen from the thin film.
A pure oxygen environment acts as a compensatory mechanism. It provides a rich reservoir of oxygen to replenish what is lost, maintaining the material's intended mass and balance.
Preserving Chemical Stoichiometry
For NMC (Nickel Manganese Cobalt) films to function correctly, the ratio of their chemical components—their stoichiometry—must be precise.
Oxygen loss disrupts this ratio. By annealing in pure oxygen, you force the material to maintain the correct chemical balance required for battery performance.
Controlling Phase Transitions
Preventing Nickel Reduction
One of the most specific dangers of oxygen deficiency is the chemical reduction of Nickel ions.
The target material requires Nickel to exist in the Ni3+ state. If the environment lacks sufficient oxygen, Ni3+ reduces to NiO (Nickel Oxide).
Avoiding the Rock-Salt Phase
When Nickel reduces to NiO, it forms a rock-salt phase. This phase is detrimental because it is electrochemically inactive compared to the desired layered structure.
A pure oxygen atmosphere suppresses this reaction, effectively blocking the formation of the unwanted rock-salt phase.
Enabling Crystallization
NMC thin films often start in an amorphous state (a disordered atomic structure).
The annealing process is intended to reorganize these atoms into a defined crystalline structure that is electrochemically active. The presence of pure oxygen facilitates this transition, ensuring the final crystal lattice is robust and capable of storing energy.
Common Pitfalls to Avoid
The Risk of Atmospheric Dilution
It may be tempting to use air (which is only ~21% oxygen) or inert gases to lower process complexity.
However, any dilution of the oxygen concentration increases the probability of Ni3+ reduction. Even minor deviations can lead to mixed-phase materials that exhibit poor capacity and cycle life.
Misinterpreting Structural Integrity
Achieving a solid film is not the same as achieving an active film.
A film annealed in low oxygen might look mechanically sound but will be electrochemically dead due to the dominance of the NiO rock-salt phase. You cannot rely on visual inspection alone; process atmosphere control is the primary safeguard for quality.
Optimizing Your Annealing Strategy
To maximize the performance of your NMC thin films, align your process parameters with your specific material goals:
- If your primary focus is Phase Purity: Ensure continuous oxygen flow to strictly prevent the reduction of Ni3+ into the inactive NiO rock-salt phase.
- If your primary focus is Electrochemical Activity: Maintain a pure oxygen environment to support the complete transition from an amorphous precursor to a functional crystalline lattice.
Control the atmosphere, and you control the fundamental quality of the cathode material.
Summary Table:
| Factor | Impact of Pure Oxygen | Risk of Oxygen Deficiency |
|---|---|---|
| Stoichiometry | Maintains precise chemical balance | Evaporative loss and chemical imbalance |
| Nickel State | Preserves essential Ni3+ oxidation state | Reduction of Ni3+ to NiO |
| Phase Structure | Ensures layered crystalline structure | Formation of inactive rock-salt phase |
| Performance | High electrochemical activity | Low capacity and poor cycle life |
Maximize the Performance of Your NMC Thin Films
Precision atmosphere control is the difference between an active cathode and an inactive rock-salt phase. KINTEK provides high-performance tube furnaces and advanced vacuum systems specifically designed to handle pure oxygen environments for delicate annealing processes.
Backed by expert R&D and world-class manufacturing, we offer customizable Tube, Muffle, Rotary, and CVD systems tailored to your lab's unique high-temperature needs. Don't compromise your material's stoichiometry with inadequate thermal equipment.
Ready to elevate your research and production? Contact KINTEK today to find the perfect customized furnace for your thin-film applications.
Visual Guide
References
- Sameer R.J. Rodrigues, Philippe M. Vereecken. Coupled Solid‐State Diffusion of Li<sup>+</sup> and O<sup>2 −</sup> During Fabrication of Ni‐Rich NMC Thin‐Film Cathodes Resulting in the Formation of Inactive Ni<sub>2</sub>O<sub>3</sub> and NiO Phases. DOI: 10.1002/admi.202400911
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What factors affect the price of a vacuum tube furnace? Key Drivers and Smart Investment Tips
- How is tantalum disulfide prepared using a tube furnace? Master the Two-Step Synthesis for High-Quality Crystals
- What is the role of a Tube Furnace in TMDC-ND preparation? Master Graphene-Decorated Nanostructure Synthesis
- How is a tube furnace designed to operate at 1200°C? Precision Engineering for Extreme Heat
- What are the primary uses of tube furnaces in academic and industrial settings? Unlock Precision Thermal Processing
- Why is a Horizontal Tube Furnace used for the torrefaction of Refuse Derived Fuel (RDF)? Boost Fuel Efficiency Now
- What is the function of a laboratory tube furnace in Ti-5Al-4W-2Fe alloy forging? Enhance Thermoplasticity & Purity
- What materials are commonly used for the heating element in tubular furnaces? Choose the Best for Your High-Temp Needs



















