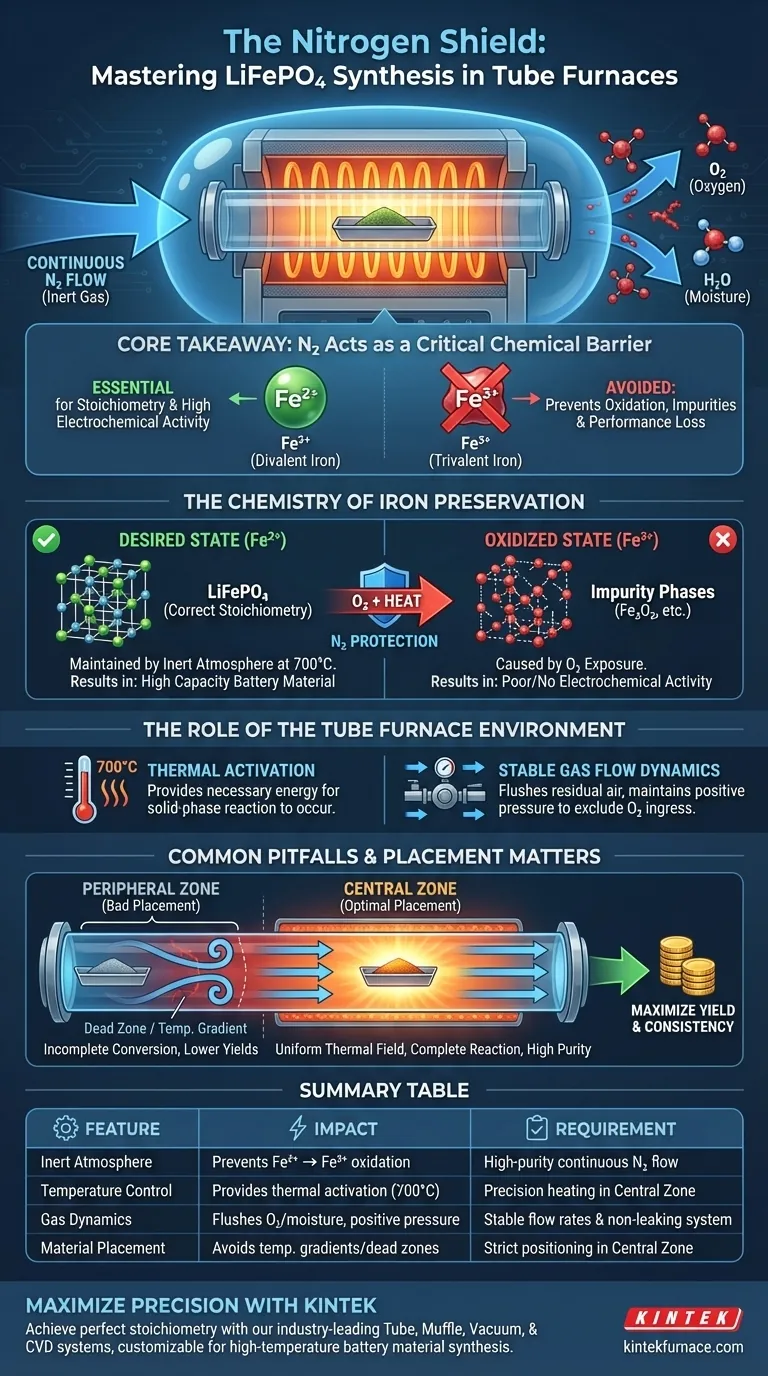
A nitrogen-protected atmosphere acts as a critical chemical barrier against oxidation. During the high-temperature solid-phase synthesis of lithium iron phosphate, specifically at temperatures reaching 700°C, the presence of oxygen is chemically destructive. A continuous nitrogen flow creates an inert environment within the atmospheric tube furnace, strictly excluding oxygen to preserve the integrity of the reactants.
Core Takeaway The fundamental purpose of the nitrogen atmosphere is to prevent divalent iron ($Fe^{2+}$) from oxidizing into trivalent iron ($Fe^{3+}$). Preserving the iron in its $+2$ state is mandatory for ensuring the final product maintains the correct stoichiometry and high electrochemical activity.

The Chemistry of Iron Preservation
Preventing Oxidation States from Shifting
The synthesis of lithium iron phosphate ($LiFePO_4$) relies on the iron remaining in a divalent state ($Fe^{2+}$).
At the requisite high temperatures of 700°C, iron is highly reactive and susceptible to oxidation. Without a protective atmosphere, ambient oxygen would rapidly convert the desirable $Fe^{2+}$ into trivalent iron ($Fe^{3+}$).
Ensuring Stoichiometric Accuracy
The transformation to $Fe^{3+}$ fundamentally alters the chemical composition of the material.
If oxidation occurs, the final product will fail to meet the required stoichiometry. This degradation directly results in a product with poor or non-existent electrochemical activity, rendering it useless for battery applications.
The Role of the Tube Furnace Environment
Providing Thermal Activation
The tube furnace provides the necessary thermal activation energy to drive the solid-phase reaction.
However, heat alone is insufficient; the furnace must simultaneously maintain a strictly controlled atmosphere. This combination allows for precise thermal control while ensuring the chemical phase purity of the product.
Stabilizing Gas Flow Dynamics
An atmospheric tube furnace is designed to provide a continuous and stable nitrogen flow.
This flow must be constant to flush out any residual air and maintain positive pressure, preventing oxygen ingress. This creates a uniform inert environment necessary for the complete crystal structure formation of the phosphate.
Common Pitfalls and Operational Trade-offs
The Danger of Peripheral Placement
While the atmosphere is critical, the physical placement of the precursor material within the furnace is equally important.
Materials placed in the peripheral areas of the tube often experience temperature gradients or gas flow "dead zones." This can lead to incomplete precursor conversion, regardless of the nitrogen flow.
Incomplete Reactions and Impurities
Failing to utilize the optimal zone of the furnace leads to unreacted residues.
Research indicates that precursors must be placed in the central zone to achieve the most uniform thermal field and gas dynamics. Placing materials outside this zone often results in the formation of impurity phases and lower overall yields.
Making the Right Choice for Your Synthesis
To ensure the success of your lithium iron phosphate synthesis, you must control both the chemistry and the physical environment.
- If your primary focus is Phase Purity: Ensure a continuous, high-purity nitrogen flow to strictly maintain the $Fe^{2+}$ oxidation state and prevent $Fe^{3+}$ formation.
- If your primary focus is Consistency and Yield: Position your precursors strictly in the central zone of the tube furnace to avoid temperature gradients and gas dead zones.
Success in this synthesis depends not just on reaching 700°C, but on maintaining a rigorous inert environment that protects the fundamental chemistry of the iron.
Summary Table:
| Feature | Impact on LiFePO4 Synthesis | Requirement for Success |
|---|---|---|
| Inert Atmosphere | Prevents $Fe^{2+}$ to $Fe^{3+}$ oxidation | High-purity continuous Nitrogen flow |
| Temperature Control | Provides thermal activation at 700°C | Precision heating in the Central Zone |
| Gas Dynamics | Flushes residual oxygen/moisture | Positive pressure & stable flow rates |
| Material Placement | Avoids temperature gradients/dead zones | Centralized positioning of precursors |
Maximize Your Synthesis Precision with KINTEK
Achieving the perfect stoichiometric balance for lithium iron phosphate requires rigorous atmospheric control and thermal uniformity. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems designed specifically for high-temperature solid-phase reactions. Backed by expert R&D and manufacturing, our furnaces are fully customizable to eliminate temperature gradients and ensure a pure inert environment for your battery materials.
Ready to elevate your lab's electrochemical yields? Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Tengshu Chen, Liyao Chen. Research on the synthesis of lithium iron phosphate using vivianite prepared from municipal sludge. DOI: 10.1038/s41598-025-16378-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are the advantages of using a high-temperature tube furnace for rGO sensor fabrication? Precision & Performance
- Why is a high-performance tube furnace required for chemical activation? Achieve Precision Pore Control at 700°C
- What are the key operational considerations when using a lab tube furnace? Master Temperature, Atmosphere & Safety
- What is the function of the pre-oxidation process conducted in a tube furnace? Stabilize Lignin for Carbon Fibers.
- How does a tube furnace contribute to the CVD of Si-SiO2 composites? Achieve Precise Nanostructure Control
- What are the continuous operating temperatures for each zone in a three-zone split tube furnace? Choose the Right Model for Your Lab
- What conditions does a tubular reactor provide for catalyst reduction? Master Platinum, Copper, and Nickel Activation
- What temperature-related capabilities make multi zone tube furnaces valuable for research? Unlock Precision Thermal Control



















