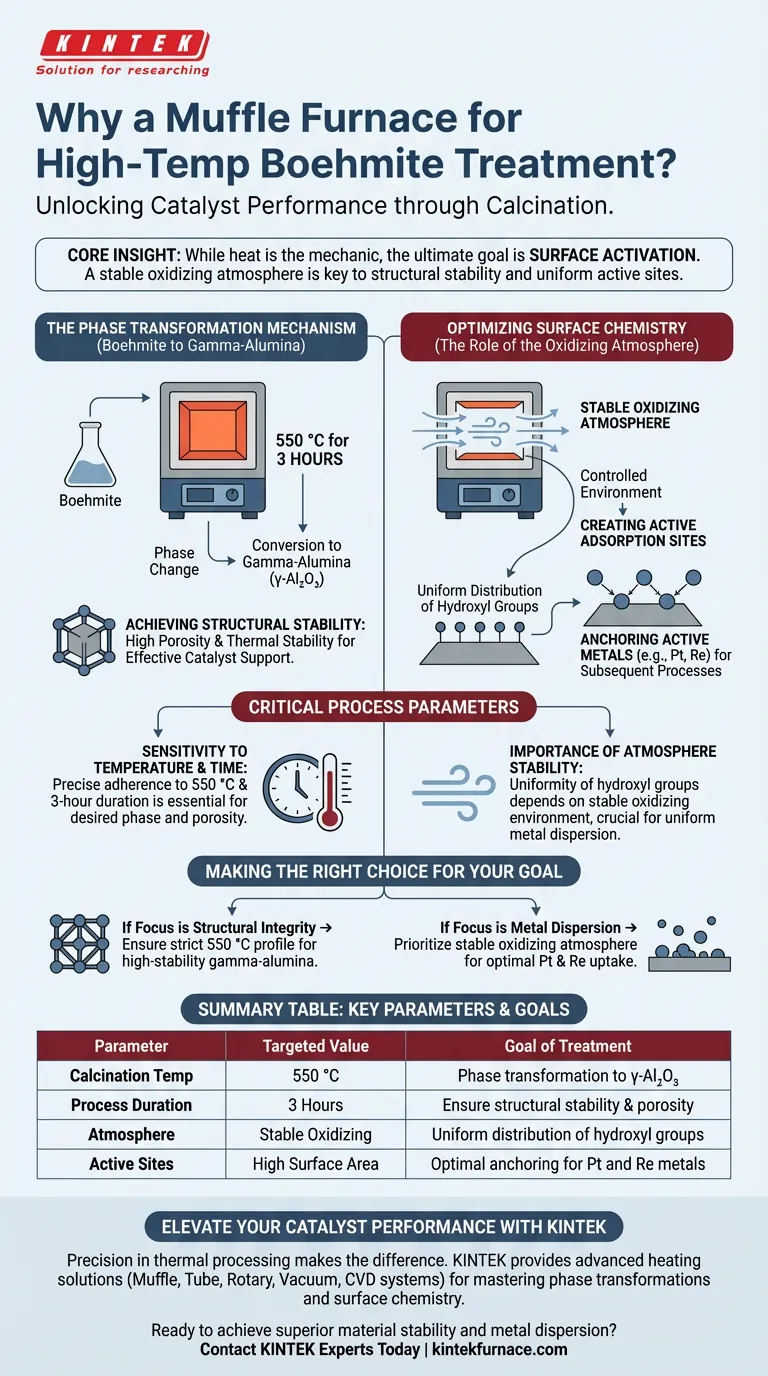
A muffle furnace is utilized primarily to execute the calcination process that transforms Boehmite into gamma-alumina ($\gamma$-Al$_2$O$_3$). By maintaining a temperature of 550 °C for three hours, the furnace drives the specific structural changes necessary to create a high-performance catalyst support.
Core Insight While heat is the mechanic, the ultimate goal is surface activation. The muffle furnace provides a stable oxidizing atmosphere that not only structurally stabilizes the material but creates a uniform distribution of surface hydroxyl groups, which are essential for anchoring active metals during later production stages.

The Phase Transformation Mechanism
Conversion to Gamma-Alumina
The primary function of the muffle furnace in this context is to thermally treat Boehmite at 550 °C for three hours.
This specific thermal exposure triggers a phase change, converting the starting Boehmite material into gamma-alumina ($\gamma$-Al$_2$O$_3$).
Achieving Structural Stability
The resulting gamma-alumina serves as a robust support material.
This calcination process ensures the support achieves high porosity and thermal stability, providing the physical backbone required for an effective industrial catalyst.
Optimizing Surface Chemistry
The Role of the Oxidizing Atmosphere
Beyond simple heating, the muffle furnace provides a controlled, stable oxidizing atmosphere.
This environment is critical for managing the chemical nature of the support's surface, ensuring it is chemically receptive to further modification.
Creating Active Adsorption Sites
The oxidizing conditions foster a uniform distribution of surface hydroxyl groups on the carrier.
These hydroxyl groups act as optimal chemical adsorption sites. They facilitate the effective anchoring of active metals, such as platinum (Pt) and rhenium (Re), during subsequent wet impregnation processes.
Critical Process Parameters
Sensitivity to Temperature and Time
The conversion of Boehmite relies on precise adherence to the 550 °C and three-hour duration parameters.
Deviating from this specific thermal profile can fail to produce the desired gamma-alumina phase or result in suboptimal porosity.
Importance of Atmosphere Stability
The uniformity of the hydroxyl groups is directly linked to the stability of the oxidizing environment.
Fluctuations in the furnace atmosphere can lead to uneven surface chemistry, which compromises the dispersion and effectiveness of the active metals later in the manufacturing cycle.
Making the Right Choice for Your Goal
To ensure your catalyst preparation yields the highest performance, consider the following focus areas:
- If your primary focus is structural integrity: Ensure the furnace maintains a strict 550 °C profile to guarantee the complete phase conversion to high-stability gamma-alumina.
- If your primary focus is metal dispersion: Prioritize the stability of the oxidizing atmosphere to maximize the uniformity of hydroxyl groups for optimal Platinum and Rhenium uptake.
Precise control over the calcination environment is the single most important factor in preparing a receptive and stable catalyst support.
Summary Table:
| Parameter | Targeted Value | Goal of Treatment |
|---|---|---|
| Calcination Temp | 550 °C | Phase transformation to $\gamma$-Al$_2$O$_3$ |
| Process Duration | 3 Hours | Ensure structural stability & porosity |
| Atmosphere | Stable Oxidizing | Uniform distribution of hydroxyl groups |
| Active Sites | High Surface Area | Optimal anchoring for Pt and Re metals |
Elevate Your Catalyst Performance with KINTEK
Precision in thermal processing is the difference between a mediocre support and a high-performance industrial catalyst. KINTEK provides the advanced heating solutions required to master phase transformations and surface chemistry.
Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems. Our laboratory high-temperature furnaces are fully customizable to meet your specific research or production needs, ensuring strict temperature uniformity and atmosphere stability for critical materials like Boehmite.
Ready to achieve superior material stability and metal dispersion?
Visual Guide
References
- Domenic Strauch, Moritz Wolf. Bimetallic platinum rhenium catalyst for efficient low temperature dehydrogenation of perhydro benzyltoluene. DOI: 10.1039/d3cy01336g
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a program-controlled muffle furnace ensure BAF polyimide film performance? Master Thermal Imidization Control
- What role does a high-temperature muffle furnace play in ZnO/CuO preparation? Master Nanocomposite Synthesis
- What is the purpose of a muffle furnace in microbiological analysis? Achieve Absolute Sterility and Precise Sample Preparation
- What are the different types of muffle furnaces? Choose the Right Furnace for Your Lab Needs
- What is the significance of using a box resistance furnace for the 900 °C sintering of high-entropy alloys?
- What role does a muffle furnace play in the post-treatment of cobalt-based catalysts? Optimize Phase Purity via Annealing
- How is the box furnace's door secured and what safety feature does it have? Ensuring Safe Operation with Robust Design
- What are the benefits of using an electric furnace for home heating? Uncover the Safe, Efficient Solution



















