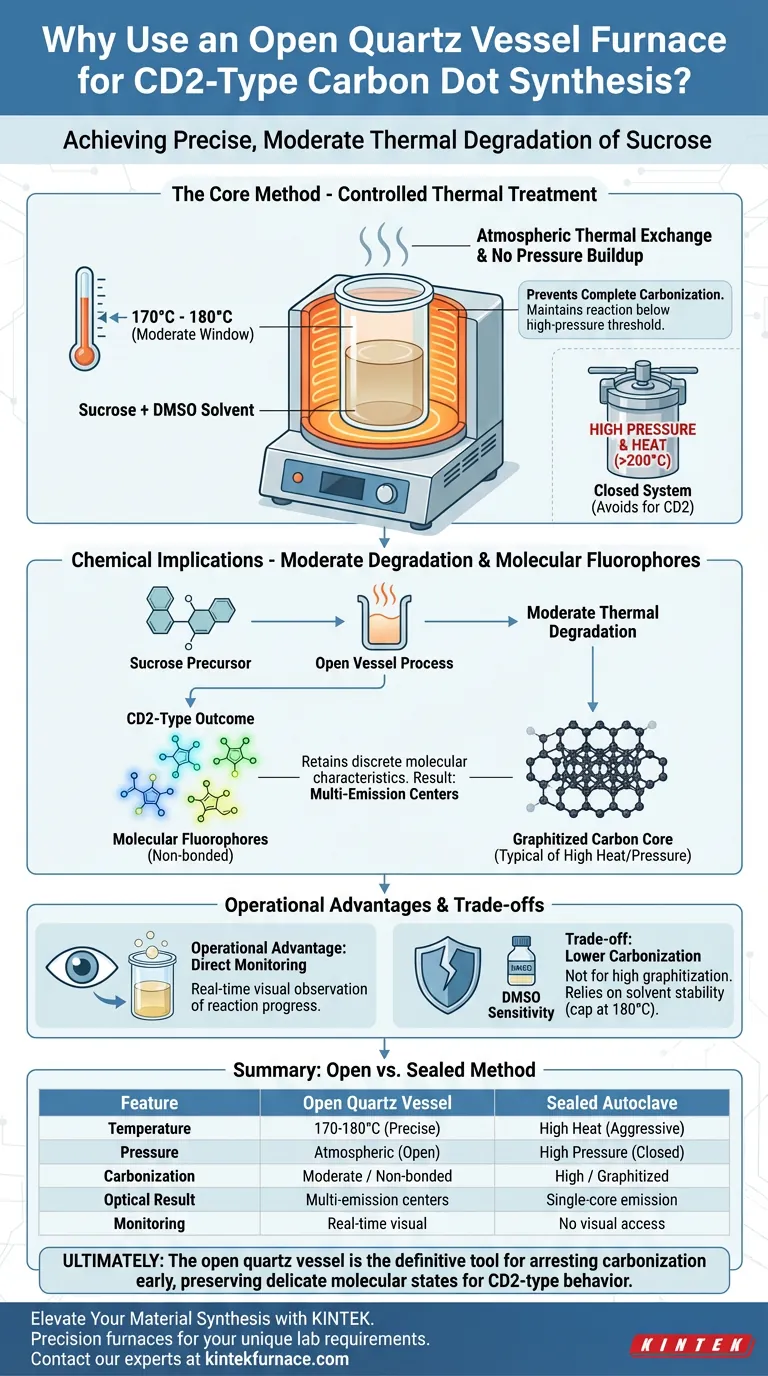
The primary reason for utilizing a laboratory furnace with an open quartz vessel is to achieve precise, moderate thermal degradation of sucrose within a specific temperature window. This setup allows researchers to maintain the reaction between 170°C and 180°C, preventing the complete carbonization that typically occurs in closed, high-pressure systems.
Core Takeaway: This open-system approach prioritizes the formation of molecular fluorophores over graphitized carbon cores. By facilitating thermal exchange and preventing pressure buildup, the method yields "non-bonded" CD2-type dots known for their unique multi-emission center characteristics.
The Role of Controlled Thermal Treatment
To understand why this specific equipment is used, you must look at the thermal requirements of CD2-type synthesis.
Targeting the 170°C to 180°C Window
The synthesis of CD2-type dots relies on a "mid-to-low" temperature strategy.
The laboratory furnace is calibrated to maintain a steady environment between 170°C and 180°C. This specific range is critical for initiating the breakdown of sucrose without supplying enough energy to force the material into a fully graphitized state.
Facilitating Thermal Exchange
An open quartz vessel allows for efficient thermal exchange between the solvent (DMSO) and the furnace environment.
Unlike a sealed autoclave, which traps heat and pressure, the open vessel ensures the solvent remains at the desired processing temperature through equilibrium with the furnace atmosphere.
Chemical Implications of the Open System
The physical setup directly dictates the chemical structure of the resulting nanomaterials.
Moderate Degradation of Sucrose
The goal of this method is moderate thermal degradation, not complete combustion or carbonization.
By using an open vessel with dimethyl sulfoxide (DMSO) as the solvent, the process gently breaks down the sucrose precursor. This controlled breakdown preserves specific chemical structures that would otherwise be destroyed in harsher environments.
Producing Molecular Fluorophores
The result of this gentle heating is the production of molecular fluorophores.
Because the reaction is not driven to full carbonization, the resulting dots are defined as "non-bonded." This means they retain discrete molecular characteristics rather than forming a unified, graphitized carbon core.
Multi-Emission Characteristics
The preservation of these molecular fluorophores endows CD2-type dots with multi-emission center characteristics.
This optical versatility is a direct result of the incomplete carbonization permitted by the open-furnace method.
Operational Advantages
Beyond the chemical outcome, the equipment offers practical benefits for reaction management.
Direct Process Monitoring
The open nature of the quartz vessel allows for direct visual monitoring of the reaction progress.
Researchers can observe color changes or physical transitions in real-time, allowing them to stop the reaction precisely when the desired degradation level is reached.
Understanding the Trade-offs
While effective for CD2-type dots, this method has limitations compared to other synthesis techniques like hydrothermal carbonization.
Lower Carbonization Degree
This method is not suitable if your goal is to create highly crystalline, graphitized carbon dots.
The open system prevents the pressure buildup required to force the carbon atoms into a tight, graphitic lattice structure.
Solvent Sensitivity
Because the vessel is open, the process relies heavily on the properties of the solvent (DMSO).
You must ensure the operating temperature does not exceed the solvent's boiling point or stability limits to prevent evaporation or hazardous fumes, necessitating the 170-180°C cap.
Making the Right Choice for Your Goal
Select your synthesis equipment based on the specific optical and structural properties you require for your carbon dots.
- If your primary focus is generating molecular fluorophores: Use the open quartz vessel and furnace method to ensure moderate degradation and multi-emission properties.
- If your primary focus is high graphitization: Avoid this method and opt for a sealed autoclave (hydrothermal method) to achieve higher pressures and carbon core formation.
Ultimately, the open quartz vessel is the definitive tool for arresting the carbonization process early, preserving the delicate molecular states required for CD2-type behavior.
Summary Table:
| Feature | Open Quartz Vessel Method | Sealed Autoclave Method |
|---|---|---|
| Temperature Range | 170°C - 180°C (Precise/Moderate) | High Heat (Aggressive) |
| Pressure Level | Atmospheric (Open system) | High Pressure (Closed) |
| Carbonization | Moderate / Non-bonded | High / Graphitized |
| Optical Result | Multi-emission centers | Single-core emission |
| Monitoring | Real-time visual observation | No visual access |
Elevate Your Material Synthesis with KINTEK
Precision is the difference between complete carbonization and the perfect molecular fluorophore. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your unique laboratory requirements.
Whether you are synthesizing CD2-type carbon dots or developing advanced nanomaterials, our high-temperature furnaces provide the thermal stability and control your research demands.
Ready to optimize your thermal processing? Contact our experts today to find the ideal furnace solution for your lab.
Visual Guide
References
- Oleg Dimitriev, A. N. Nazarov. Photoluminescence quantum yield of carbon dots: emission due to multiple centers <i>versus</i> excitonic emission. DOI: 10.1039/d4na00033a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why are electric furnaces considered a quieter heating option? Silent Operation Explained
- How is a high-temperature muffle furnace utilized to evaluate the oxidation resistance of Cr2AlC ceramics?
- What features make muffle furnaces easy to operate? Discover Key Ease-of-Use Features
- Why is temperature range important when selecting a muffle furnace? Ensure Process Success and Equipment Longevity
- What contributes to the stability of box type high-temperature resistance furnaces? Key Factors for Reliable Thermal Performance
- What reaction environment must a muffle furnace or tube furnace provide for g-C3N4? Master Thermal Polymerization
- What is the function of a high-temperature muffle furnace? Master Eggshell Adsorbent Activation
- What role does a laboratory oven play in determining the porosity of FOPC? Ensuring Precision in Material Density



















