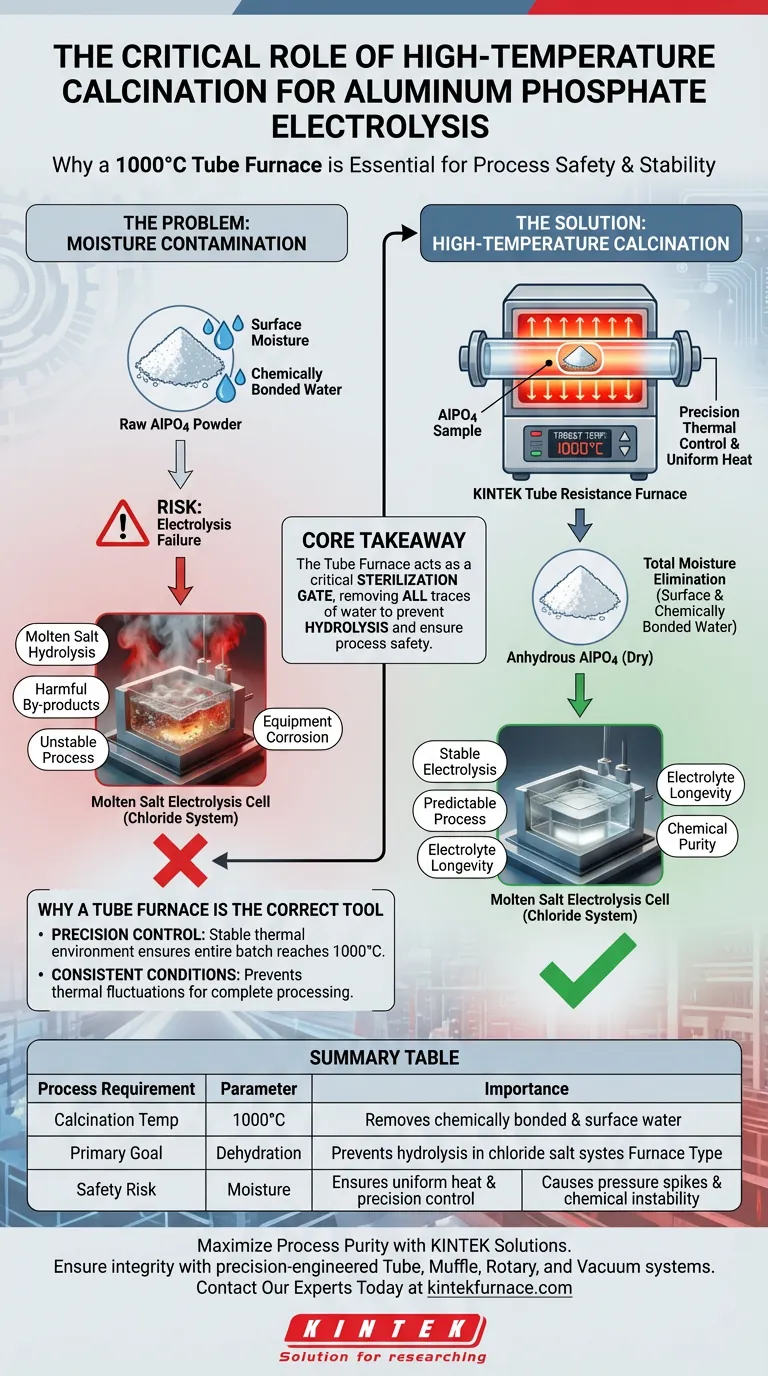
The primary function of the high-temperature tube furnace in this context is total moisture elimination to ensure process safety. Specifically, the furnace is used to calcine aluminum phosphate (AlPO4) at 1000°C, a temperature critical for stripping away not just surface moisture, but also chemically bonded water. This step is a mandatory prerequisite to prevent catastrophic chemical instability in the subsequent electrolysis stage.
Core Takeaway The success of molten salt electrolysis relies entirely on the purity of the precursor material. The tube furnace acts as a critical sterilization gate, removing all traces of water to prevent the hydrolysis of chloride salts, which would otherwise generate harmful by-products and destabilize the electrolytic system.

The Critical Role of Moisture Elimination
Targeting Two Types of Water
Standard drying methods are insufficient for preparing aluminum phosphate for this process. Simple heating removes physically adsorbed moisture (surface wetness).
However, you must use a high-temperature furnace to reach 1000°C to break the stronger bonds of chemically bonded water. Without this extreme heat, water remains trapped within the molecular structure of the AlPO4.
Preventing Molten Salt Hydrolysis
The electrolysis of AlPO4 takes place in a chloride salt system. These systems are chemically intolerant to water.
If moisture is introduced into the molten bath, it triggers hydrolysis. This reaction decomposes the salt, creating harmful by-products that contaminate the electrolyte and alter the electrochemistry of the cell.
Ensuring Process Stability
Stability is the ultimate goal of the pre-calcination step. By ensuring the input material is completely anhydrous, you eliminate variables that cause erratic reaction kinetics.
This allows the electrolysis process to proceed predictably, maintaining the precise conditions required for material separation or deposition.
Why a Tube Furnace is the Correct Tool
Precision Thermal Control
While the primary goal is dehydration, the quality of the heat source matters. A precision tube resistance furnace provides a highly stable thermal environment.
This stability ensures that the entire batch of AlPO4 reaches the target temperature of 1000°C uniformly, leaving no pockets of uncalcined material.
Maintaining Consistent Conditions
Supplementary data from similar electrochemical experiments confirms that tube furnaces are essential for maintaining constant temperatures (e.g., 950°C to 1323 K in other systems).
This precision prevents thermal fluctuations that could lead to incomplete processing or phase changes that negatively impact the dissolution of oxides in the salt melt.
Understanding the Trade-offs
The Risk of Under-Calcination
Attempting to save energy by lowering the calcination temperature below 1000°C is a common pitfall.
If the temperature is insufficient, chemically bonded water will remain. When this "wet" material hits the molten salt, it will release water vapor instantly, leading to dangerous pressure spikes and chemical degradation of the bath.
Energy vs. Purity
High-temperature calcination is energy-intensive. However, this energy cost is a necessary trade-off for chemical purity.
Skimping on this pre-treatment step inevitably leads to higher costs downstream due to ruined electrolytes, corroded equipment from hydrolysis by-products, and failed electrolysis runs.
Making the Right Choice for Your Goal
To maximize the efficiency of your aluminum phosphate electrolysis, apply the following principles:
- If your primary focus is Process Safety: Ensure your calcination protocol strictly holds the material at 1000°C to guarantee the complete removal of chemically bonded water.
- If your primary focus is Electrolyte Longevity: Prioritize the use of a precision tube furnace to prevent moisture-induced hydrolysis, which degrades the expensive chloride salt mixture.
Ultimately, the tube furnace is not just a heating device; it is a purification tool that protects the chemical integrity of your entire electrolysis system.
Summary Table:
| Process Requirement | Parameter | Importance |
|---|---|---|
| Calcination Temp | 1000°C | Removes chemically bonded & surface water |
| Primary Goal | Dehydration | Prevents hydrolysis in chloride salt systems |
| Furnace Type | Tube Resistance | Ensures uniform heat & precision control |
| Safety Risk | Moisture | Causes pressure spikes & chemical instability |
Maximize Process Purity with KINTEK Solutions
Ensure the integrity of your electrolysis with precision-engineered thermal equipment. KINTEK provides industry-leading Tube, Muffle, Rotary, and Vacuum systems designed to meet the rigorous demands of high-temperature calcination.
Our expert R&D and manufacturing teams offer customizable lab furnaces tailored to your specific material needs—helping you eliminate chemical instability and protect your equipment from hydrolysis damage.
Ready to optimize your lab's thermal processing?
Visual Guide
References
- Yuxiang Zhong, Xiao Yang. Extracting White Phosphorus from AlPO<sub>4</sub> through Molten Salt Processing. DOI: 10.5796/electrochemistry.24-69001
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What critical experimental conditions does a tube furnace provide for carbonizing PI-COFs? Master Thermal Precision
- What role does a tube furnace play in the preparation of biochar-filled PVC composite precursors? Expert Synthesis Guide
- What types of heating elements are commonly used in drop tube furnaces? Find the Right Element for Your Temperature Needs
- Can you provide an example of a material prepared using a tube furnace? Discover YBa₂Cu₃O₇ Synthesis
- How does a vertical tube furnace ensure effective hydrogen reduction reactions? Optimize Rare Earth Tailing Processing
- What are the safety and usability features of tube furnaces? Essential for Precise Material Processing
- What are the main applications of a drop tube furnace? Unlock Insights in Energy and Materials Research
- What are the specialized functions of a high-temperature tube furnace in the final sintering of proton ceramics?



















