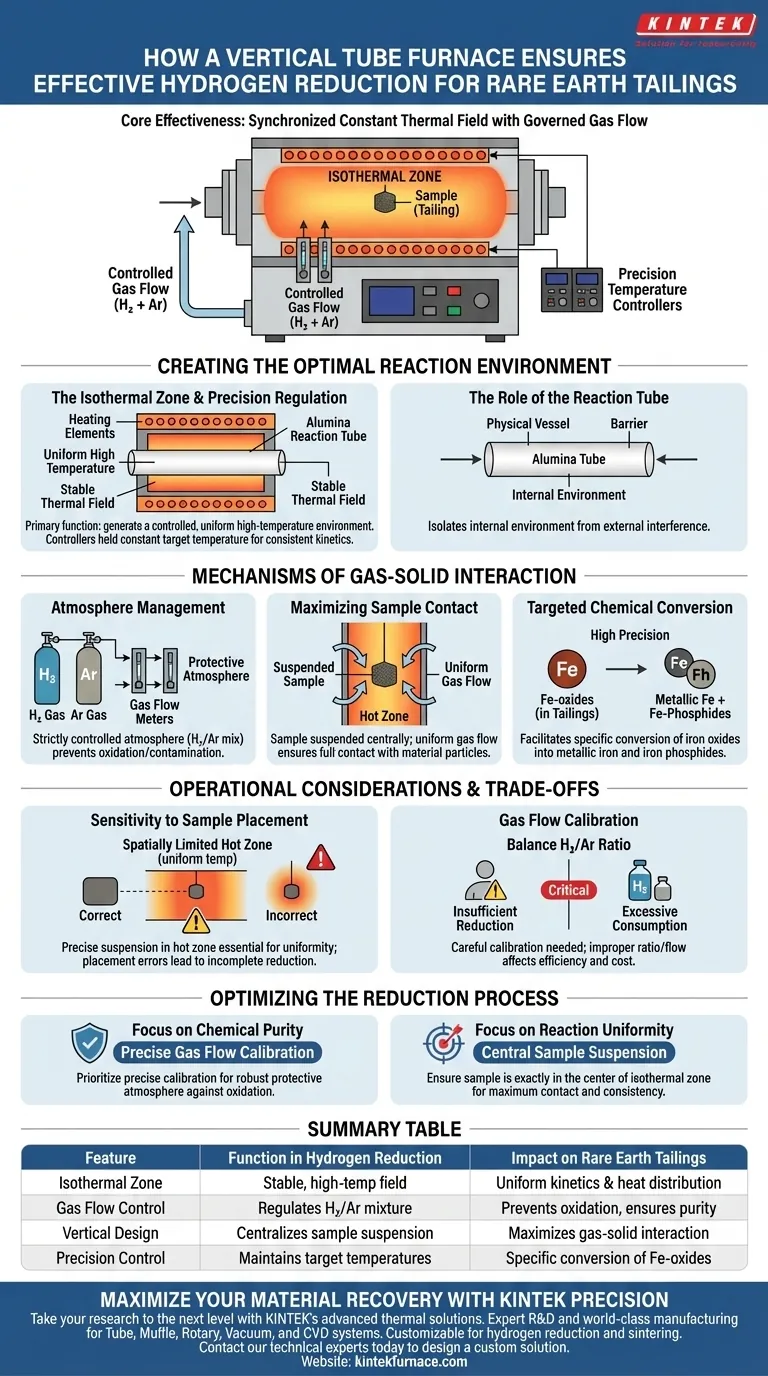
A vertical tube furnace ensures effective hydrogen reduction by creating a highly stable, high-temperature isothermal zone. Through the use of precision temperature controllers and gas flow meters, the system maintains a consistent atmosphere of hydrogen and argon mixtures. This setup forces the reducing gas to make full contact with tailing samples suspended in the hot zone, allowing for the precise conversion of iron oxides into metallic iron and iron phosphides.
The core effectiveness of this apparatus lies in its ability to synchronize a constant thermal field with a governed gas flow, ensuring uniform reduction while preventing external contamination.

Creating the Optimal Reaction Environment
The Isothermal Zone
The primary function of the vertical tube furnace is to generate a controlled high-temperature environment known as the isothermal zone.
This zone serves as the core arena for chemical reduction, ensuring that the temperature remains uniform across the sample.
Precision Temperature Regulation
To maintain this stability, the system integrates high-precision temperature controllers with the furnace's heating elements.
This technology allows the furnace to hold a constant target temperature, which is essential for consistent reaction kinetics during the reduction process.
The Role of the Reaction Tube
Typically consisting of materials like alumina, the vertical tube acts as the physical vessel for the reaction.
It isolates the internal environment from the outside world, creating a distinct thermal field where the reduction can occur without interference.
Mechanisms of Gas-Solid Interaction
Atmosphere Management
Effective reduction requires a strictly controlled atmosphere, achieved by using gas flow meters to regulate mixtures of hydrogen and argon.
This atmosphere acts as a protective barrier, preventing unwanted reactions such as oxidation or external contamination during the process.
Maximizing Sample Contact
The design allows for tailing samples to be suspended directly within the hot zone.
Because the gas flow is uniform and the sample is positioned centrally, the reducing gas makes full contact with the material particles.
Targeted Chemical Conversion
This high level of contact and control facilitates the specific conversion of iron oxides found in the tailings.
Depending on the parameters set, the furnace enables the transformation of these oxides into metallic iron and iron phosphides with high precision.
Operational Considerations and Trade-offs
Sensitivity to Sample Placement
While the furnace provides a stable isothermal zone, this zone is spatially limited.
Samples must be suspended precisely within the "hot zone" to ensure uniformity; placing them too high or too low may result in incomplete reduction due to temperature gradients.
Gas Flow Calibration
Achieving the correct balance of hydrogen and argon is critical but requires careful calibration.
An improper ratio or flow rate can lead to insufficient reduction or, conversely, excessive gas consumption without added benefit.
Optimizing the Reduction Process
To maximize the efficiency of your hydrogen reduction experiments, consider the following specific strategies:
- If your primary focus is Chemical Purity: Prioritize the precise calibration of gas flow meters to maintain a robust protective atmosphere against oxidation.
- If your primary focus is Reaction Uniformity: Ensure the sample holder is suspended exactly in the center of the isothermal zone to maximize gas contact and temperature consistency.
By mastering the balance between thermal stability and gas dynamics, you turn the furnace from a simple heater into a precision instrument for chemical engineering.
Summary Table:
| Feature | Function in Hydrogen Reduction | Impact on Rare Earth Tailings |
|---|---|---|
| Isothermal Zone | Provides a stable, high-temperature field | Ensures uniform reaction kinetics and heat distribution |
| Gas Flow Control | Regulates Hydrogen/Argon mixture | Prevents oxidation and ensures consistent chemical purity |
| Vertical Design | Centralizes sample suspension | Maximizes gas-solid interaction for efficient conversion |
| Precision Control | Maintains target temperatures | Enables specific conversion of iron oxides into metallic iron |
Maximize Your Material Recovery with KINTEK Precision
Take your chemical engineering and material research to the next level with KINTEK’s advanced thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique hydrogen reduction and sintering requirements.
Whether you are processing rare earth tailings or developing advanced ceramics, our systems provide the thermal stability and atmospheric control you need for repeatable results. Contact our technical experts today to design a custom furnace solution that optimizes your lab’s efficiency and research outcomes.
Visual Guide
References
- Deddy C. Nababan, Sujeong Lee. Reduction of Iron Contained in Goethite-Rich Rare Earth Tailings by Hydrogen Gas. DOI: 10.1007/s11663-025-03826-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- How does the horizontal design of these furnaces benefit large volume processing? Boost Efficiency and Uniformity
- What types of gases can be introduced into the 3-Zone tube furnace? Optimize Your Process with the Right Atmosphere
- What is the role of a Tube Furnace or Rotary Furnace in hydrogen reduction roasting? Optimize Lithium Recovery Efficiency.
- What is a vertical tube furnace? Leverage Gravity for Superior Material Processing
- What atmospheric control features do horizontal tube furnaces offer? Enhance Precision in Material Processing
- What is the role of a three-zone tube furnace in the synthesis of single-crystal V2O5 nanosheets? Expert Insights
- What thermal processes can tube furnaces perform? Achieve Precise High-Temperature Control for Your Lab
- What benefits does a horizontal tube furnace offer? Achieve Precise Heat Control and Easy Access for Your Lab



















