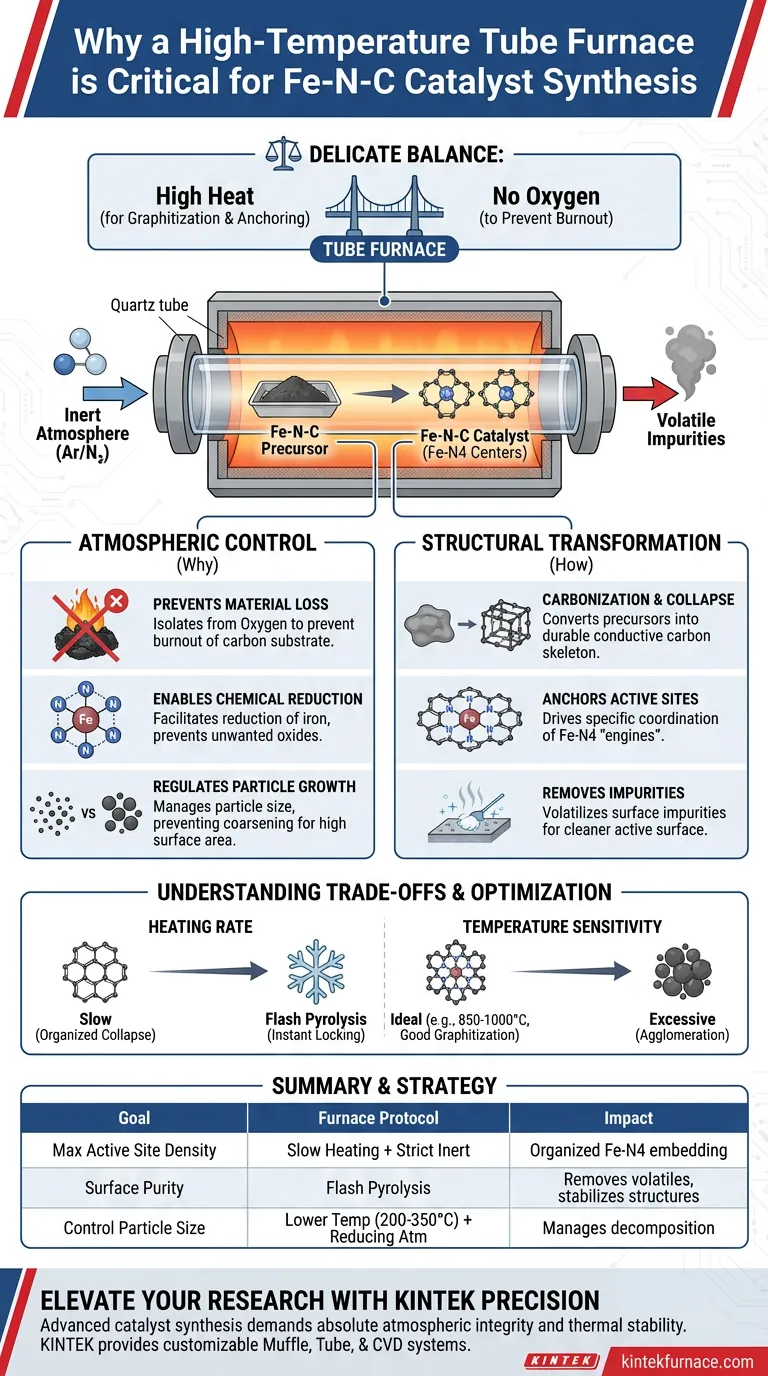
A high-temperature tube furnace is the critical enabling tool for Fe-N-C synthesis because it provides a strictly controlled, inert thermal environment necessary for carbonization without combustion. By heating precursors from room temperature to over 850°C under high-purity argon, the furnace drives the chemical coordination of iron and nitrogen atoms while preventing the oxidative burnout of the carbon substrate.
Core Takeaway The synthesis of Fe-N-C catalysts relies on a delicate balance: achieving high enough temperatures to graphitize carbon and anchor metal atoms, while totally excluding oxygen to prevent the material from turning into ash. The tube furnace bridges this gap by maintaining a sealed, inert atmosphere during extreme thermal processing.
The Necessity of Atmospheric Control
Preventing Material Loss
The primary function of the tube furnace is to isolate the sample from atmospheric oxygen.
Fe-N-C precursors are carbon-based; without an inert gas blanket (typically Argon or Nitrogen), heating them to synthesis temperatures (850°C–1000°C) would simply burn the carbon away.
Enabling Chemical Reduction
Beyond simple protection, the controlled atmosphere allows for active chemical reduction.
By introducing reducing gases like hydrogen or maintaining a strict inert environment, the furnace facilitates the reduction of iron species. This precise control prevents the formation of unwanted oxides and ensures the iron is chemically available to coordinate with nitrogen.
Regulating Particle Growth
The specific environment within the tube allows for the management of particle size during thermal treatment.
Precise atmospheric conditions prevent the over-coarsening of sub-nanometer particles. This ensures that the metal atoms remain dispersed rather than clumping together, which is vital for maintaining high catalytic surface area.
Driving Structural Transformation
Carbonization and Framework Collapse
High temperatures are required to convert soft precursors, such as ZIF-8 frameworks or biomass, into durable conductive carbon.
Under controlled heating (e.g., 3°C/min), the furnace induces the collapse and reorganization of these frameworks. This process embeds boron, nitrogen, and iron elements into a newly formed, robust carbon skeleton.
Anchoring Active Sites
The heat treatment is not just about structure; it is about atomic engineering.
The thermal energy drives the specific coordination of metal atoms with nitrogen atoms. This creates the Fe-N4 active centers—the "engines" of the catalyst—stabilizing them within the graphitic lattice.
Removal of Impurities
High-temperature processing effectively cleans the catalyst surface.
Thermal treatment creates thermodynamic pathways that volatilize surface impurities. This leaves behind a cleaner active surface, directly enhancing the material's initial catalytic activity.
Understanding the Trade-offs
Heating Rate Variables
The method of heating within the furnace dictates the final morphology of the catalyst.
A slow, constant heating rate facilitates the organized collapse of precursors like ZIF-8 into hollow structures. In contrast, "flash pyrolysis" (rapid thermal shock at 800°C) is used to instantaneously lock in atomic structures and remove volatile impurities, though it requires different handling.
Temperature Sensitivity
Ideally, higher temperatures improve graphitization, but there is a distinct upper limit.
While temperatures around 1000°C improve conductivity and chemical coordination, excessive heat can lead to the agglomeration of single atoms into larger, less active nanoparticles. The tube furnace provides the stability (e.g., keeping a constant 350°C or 700°C) required to navigate this narrow optimization window.
Making the Right Choice for Your Goal
To maximize the performance of your Fe-N-C catalyst, align your furnace protocols with your specific structural requirements:
- If your primary focus is maximizing active site density: Prioritize slow heating rates and strict inert atmospheres to facilitate the organized embedding of Fe-N4 centers into the carbon framework.
- If your primary focus is surface purity and immediate activity: Utilize flash pyrolysis techniques to thermally shock the sample, instantly removing volatile impurities and stabilizing atomic structures.
- If your primary focus is controlling particle size: Use lower temperature ranges (e.g., 200°C - 350°C) with reducing atmospheres to manage precursor decomposition and prevent particle coarsening.
Success in Fe-N-C synthesis is not just about applying heat; it is about the precision of the atmosphere that protects the chemistry while that heat does its work.
Summary Table:
| Synthesis Requirement | Role of Tube Furnace | Impact on Catalyst Quality |
|---|---|---|
| Atmospheric Control | Isolates sample from Oxygen using Ar/N₂ | Prevents oxidative burnout and carbon loss |
| Structural Carbonization | Controlled heating (e.g., 3°C/min) | Converts precursors into conductive carbon frameworks |
| Active Site Anchoring | Precise high-temp thermal energy | Drives formation of Fe-N4 coordination centers |
| Purity Management | Volatilization of impurities | Cleans active surfaces for higher initial activity |
| Morphology Control | Variable heating rates/Flash pyrolysis | Manages particle size and prevents agglomeration |
Elevate Your Material Research with KINTEK Precision
Advanced catalyst synthesis like Fe-N-C atomic engineering requires more than just heat; it demands absolute atmospheric integrity and thermal stability. KINTEK provides world-class laboratory solutions, including high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all meticulously designed to meet the rigorous demands of modern R&D.
Whether you are scaling up production or optimizing single-atom catalysts, our expert-backed manufacturing ensures your equipment is fully customizable to your unique research needs.
Ready to achieve superior synthesis results?
Contact KINTEK Today to Discuss Your Custom Furnace Solution
Visual Guide
References
- Davide Menga, Michele Piana. On the Stability of an Atomically‐Dispersed Fe−N−C ORR Catalyst: An <i>In Situ</i> XAS Study in a PEMFC. DOI: 10.1002/celc.202400228
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the primary functions of a high-precision tube resistance furnace? Optimize Chloride-Doped Composite Synthesis
- In which applications are split tube furnaces commonly used? Essential for Precise Thermal Processes in Research and Industry
- What advantages do tube furnaces offer for research applications? Unlock Precision in Atmosphere and Temperature Control
- What is the function of a laboratory tube furnace in Ti-5Al-4W-2Fe alloy forging? Enhance Thermoplasticity & Purity
- Why is a tube furnace with an argon atmosphere required for sintering SS316L foam? Protect Your Material Integrity
- What critical reaction conditions are provided by a tube furnace for NiS2 synthesis? Achieve Pure Phase Results
- What is the use of a quartz tube furnace? For High-Purity, Observable Material Processing
- What role do tube furnaces play in the new energy and lithium materials industry? Essential for Precision Thermal Processing



















