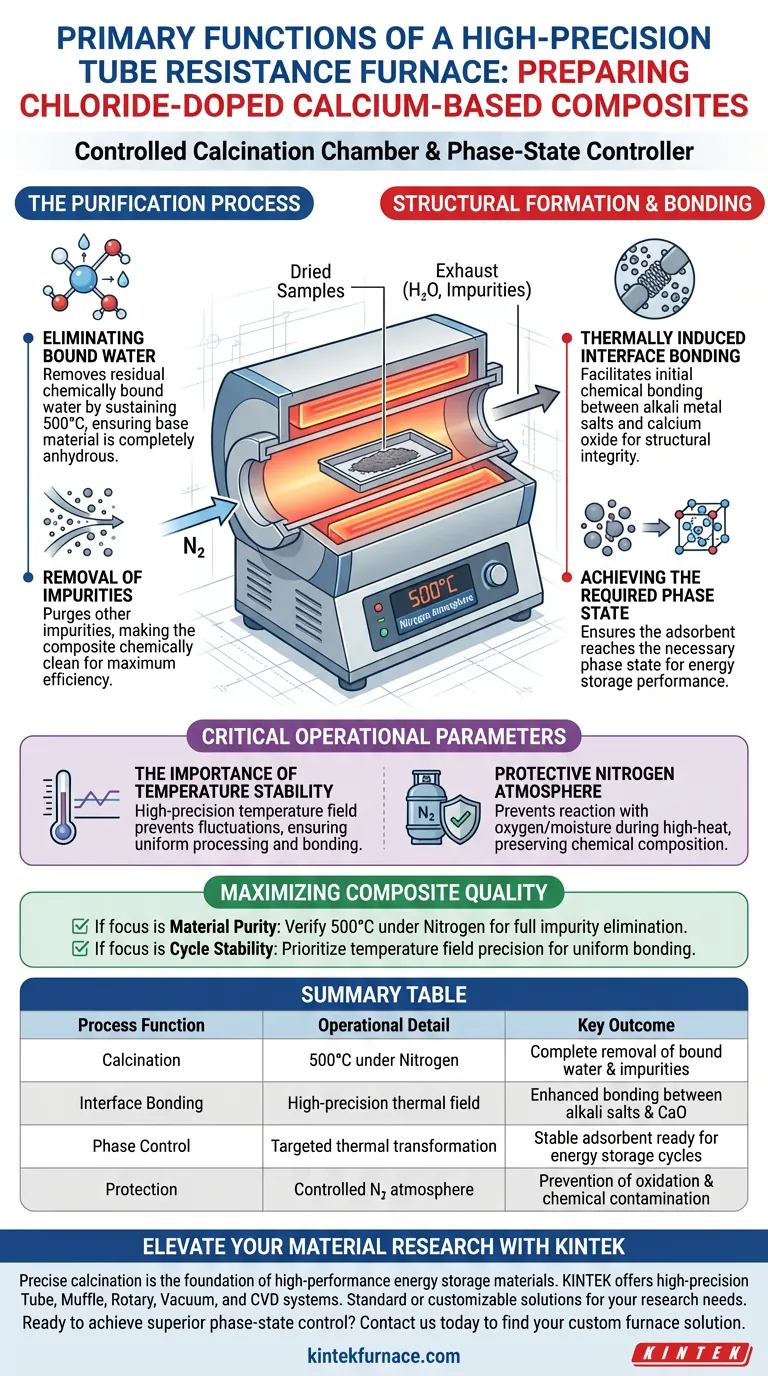
The primary function of a high-precision tube resistance furnace in this context is to act as a controlled calcination chamber. Operating at 500°C under a protective nitrogen environment, it transforms dried samples into functional adsorbents by removing residual chemically bound water and impurities. Furthermore, it drives the critical thermal bonding required between alkali metal salts and calcium oxide to prepare the material for energy storage.
The furnace is not simply a drying tool; it is a phase-state controller. Its ability to maintain a stable high-temperature field is the deciding factor in establishing the necessary interface bonding and purity required for the composite to function effectively in energy storage cycles.

The Purification Process
Eliminating Bound Water
The furnace goes beyond simple drying. It targets residual chemically bound water that standard drying processes cannot remove.
By sustaining a temperature of 500°C, the furnace forces the release of these tightly held water molecules. This ensures the base material is completely anhydrous before use.
Removal of Impurities
In addition to water, the calcination process purges other impurities from the composite.
This purification step is essential for maximizing the efficiency of the material. It ensures that the chloride-doped calcium-based composite is chemically clean prior to operation.
Structural Formation and Bonding
Thermally Induced Interface Bonding
The most complex function of the furnace is facilitating chemical changes at the microscopic level.
The heat induces initial bonding at the interface between the alkali metal salts and the calcium oxide. This bonding is critical for the structural integrity of the composite.
Achieving the Required Phase State
The composite cannot function as an energy storage medium in its raw state.
The furnace ensures the adsorbent reaches the required phase state necessary for performance. This transformation must occur specifically before the material enters its first energy storage cycle.
Critical Operational Parameters
The Importance of Temperature Stability
The term "high-precision" refers to the furnace's ability to provide a stable high-temperature field.
Fluctuations in temperature could lead to incomplete bonding or uneven phase changes. Precision ensures the entire sample is processed uniformly.
Protective Nitrogen Atmosphere
The process is conducted under a protective nitrogen environment.
This prevents the sample from reacting with oxygen or moisture in the air during the high-heat phase. It preserves the chemical composition of the doped calcium oxide during its vulnerable calcination stage.
Maximizing Composite Quality
To ensure the successful preparation of your material, consider these key objectives:
- If your primary focus is Material Purity: Verify that the furnace maintains 500°C under nitrogen for sufficient time to fully eliminate chemically bound water and impurities.
- If your primary focus is Cycle Stability: Prioritize the precision of the temperature field to guarantee uniform bonding between the alkali salts and calcium oxide.
Precision in the calcination step is the foundation of reliable energy storage performance.
Summary Table:
| Process Function | Operational Detail | Key Outcome |
|---|---|---|
| Calcination | 500°C under Nitrogen | Complete removal of bound water and impurities |
| Interface Bonding | High-precision thermal field | Enhanced bonding between alkali salts and CaO |
| Phase Control | Targeted thermal transformation | Stable adsorbent ready for energy storage cycles |
| Protection | Controlled N2 atmosphere | Prevention of oxidation and chemical contamination |
Elevate Your Material Research with KINTEK
Precise calcination is the foundation of high-performance energy storage materials. Backed by expert R&D and manufacturing, KINTEK offers high-precision Tube, Muffle, Rotary, Vacuum, and CVD systems designed to maintain the stable high-temperature fields your research demands. Whether you need a standard solution or a system fully customizable for unique needs, our lab furnaces provide the atmospheric control and thermal uniformity essential for chloride-doped composite synthesis.
Ready to achieve superior phase-state control? Contact us today to find your custom furnace solution.
Visual Guide
References
- Dehao Kong, Zhihui Wang. Enhancement of Thermochemical Energy Storage by Alkali Metal Chloride Salts-Doped Ca-Based Sorbents: A Combined DFT and Experimental Study. DOI: 10.3390/molecules29246058
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- How does a laboratory horizontal tube furnace contribute to TiO2@C synthesis? Master Thermal Treatment Stages
- How does high-temperature tube furnace programmed control influence porous carbon? Expert Pore Geometry Insights
- What is the primary role of a tube furnace in the evaluation of cable material smoke acidity? Achieve Precise Testing
- Why is precise heating rate control in a high-temperature tube furnace critical for HyDR? Master Reduction Kinetics
- What types of applications are tube furnaces suitable for? Ideal for Precise Thermal Processing in Labs
- How does the design of tube furnaces ensure uniform heating? Master Precision with Multi-Zone Control
- How does a tube furnace facilitate the annealing process for Antimony-doped ZnSe and PbSe thin films? Key Performance Tips
- What are the key benefits of using split tube furnaces? Unlock Superior Access and Control for Your Lab



















