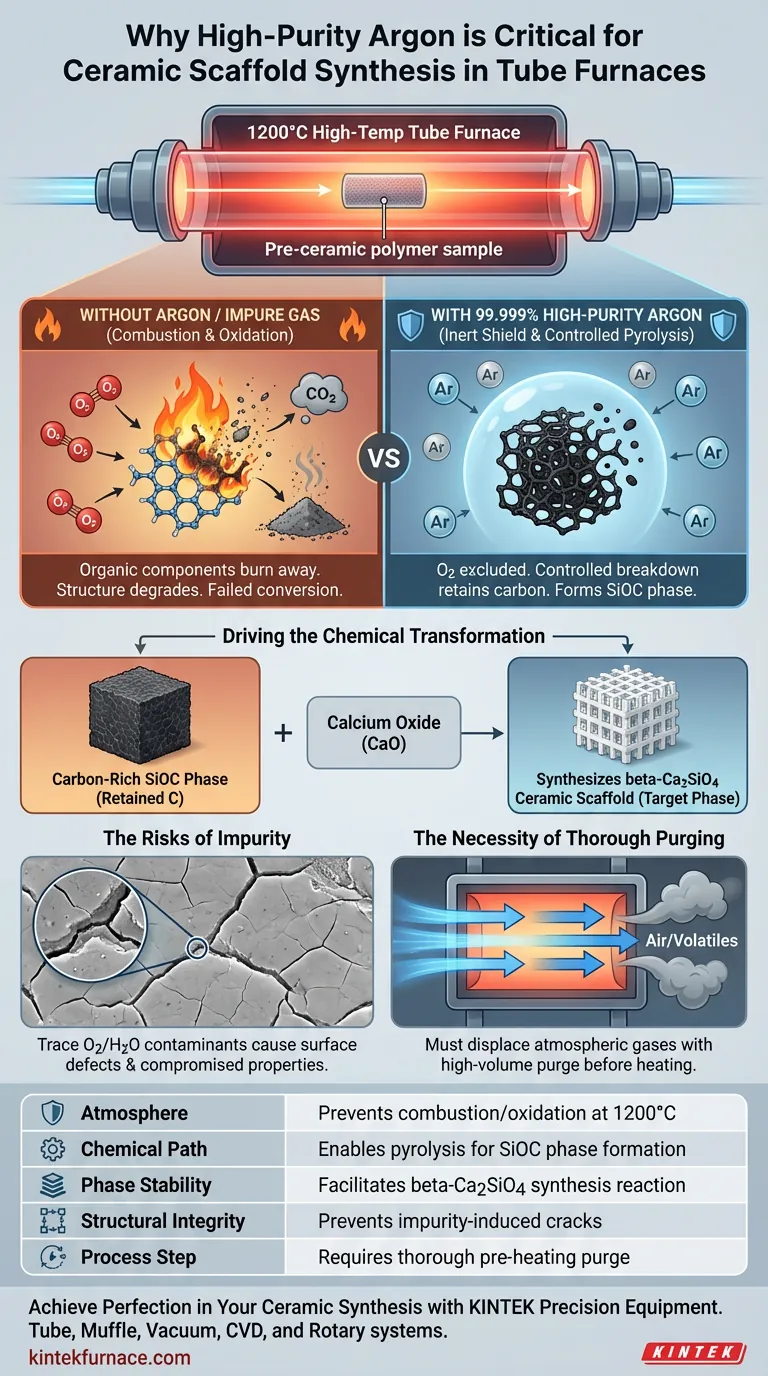
High-purity argon serves as both a chemical shield and a reaction enabler during the high-temperature conversion of pre-ceramic polymers. In a 1200°C tube furnace, this inert environment is critical to prevent the combustion of organic materials and to guide the complex chemical transformation of silicone resins into precise ceramic scaffolds.
The use of 99.999% pure argon is not merely a protective measure; it is a chemical requirement. It facilitates the controlled pyrolysis of organic components into a carbon-rich SiOC phase, which is the essential precursor for synthesizing beta-Ca2SiO4 ceramics.

The Role of Inert Atmosphere in Pyrolysis
Preventing Oxidation and Combustion
The primary function of high-purity argon is to create a completely inert environment. At sintering temperatures of 1200°C, the presence of even trace amounts of oxygen would cause the ceramic components to oxidize rapidly.
Instead of converting into a stable ceramic structure, the organic components of the pre-ceramic silicone resin would simply burn away. Argon prevents this degradation, ensuring the material retains its structural integrity during heating.
Facilitating Controlled Decomposition
The conversion process relies on pyrolysis, which is the thermal decomposition of materials in the absence of oxygen.
By maintaining an oxygen-free atmosphere, the argon allows the organic parts of the resin to break down predictably. This controlled breakdown is distinct from combustion and is vital for retaining specific elements within the matrix.
Driving the Chemical Transformation
Formation of the SiOC Phase
The specific goal of this atmosphere is to generate a carbon-rich Silicon Oxycarbide (SiOC) phase.
Because the argon protects the carbon from reacting with oxygen (which would form CO2 gas and escape), the carbon remains trapped within the ceramic structure. This retention is critical for the next stage of the reaction.
Synthesizing the Target Ceramic
The retained carbon-rich SiOC phase acts as a reactant. It interacts with decomposed calcium oxide within the matrix.
This specific reaction path, enabled only by the inert atmosphere, produces the final target phase: beta-Ca2SiO4 ceramics. Without the argon environment, this chemical pathway would be disrupted, and the desired ceramic scaffold would not form.
Understanding the Risks and Trade-offs
The Consequence of Gas Impurity
Using argon with purity lower than 99.999% is a common point of failure.
Trace impurities, such as water vapor or residual oxygen, act as contaminants. These reactive elements can alter the surface chemistry of the scaffold or lead to the formation of unwanted oxides, compromising the mechanical properties of the final ceramic.
The Necessity of Thorough Purging
Simply flowing gas during heating is insufficient; the environment must be established before the temperature rises.
The furnace chamber requires high-volume purging (e.g., high flow rates for extended durations) to physically displace atmospheric gases. Failing to remove these volatiles creates a "pseudo-inert" environment that inevitably leads to material degradation.
Making the Right Choice for Your Goal
To ensure successful conversion of pre-ceramic polymers, consider the following based on your specific objectives:
- If your primary focus is Phase Purity: Ensure your argon source is certified 99.999% pure to prevent side reactions that inhibit beta-Ca2SiO4 formation.
- If your primary focus is Structural Integrity: Implement a rigorous pre-heating purge protocol to eliminate water vapor that could cause cracks or ablation during the carbonization phase.
Strict atmosphere control is the difference between a high-performance ceramic scaffold and a degraded, oxidized failure.
Summary Table:
| Feature | Role of High-Purity Argon (99.999%) |
|---|---|
| Atmosphere | Prevents combustion and oxidation of organic components at 1200°C. |
| Chemical Path | Enables pyrolysis to form the critical carbon-rich SiOC phase. |
| Phase Stability | Facilitates the specific reaction path for beta-Ca2SiO4 synthesis. |
| Structural Integrity | Prevents trace impurities (O2/H2O) from causing surface cracks or ablation. |
| Process Step | Requires thorough pre-heating purging to displace atmospheric gases. |
Achieve Perfection in Your Ceramic Synthesis
Don't let atmospheric contamination ruin your complex pyrolysis processes. KINTEK provides the precision equipment necessary for advanced material science. Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Tube, Muffle, Vacuum, CVD, and Rotary systems—all fully customizable to meet your specific temperature and gas purity requirements.
Ensure your research yields high-performance ceramic scaffolds with the industry's most reliable high-temperature furnaces. Contact KINTEK today to discuss your unique project needs.
Visual Guide
References
- Joelle El Hayek, Chrystelle Salameh. 3D printed bioactive calcium silicate ceramics as antibacterial scaffolds for hard tissue engineering. DOI: 10.1039/d3ma01088k
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the common applications of three-zone furnaces? Achieve Precise Thermal Control for Sensitive Processes
- What optional features are available for tube furnaces? Enhance Your Materials Processing with Precision Control
- How does a laboratory tube furnace facilitate the control of pore structures? Master Precision Porous Carbon Synthesis
- Why is a tube furnace with high-precision control required for annealing platinum-decorated ruthenium catalysts?
- What technological requirements affect tube furnace design? Key Factors for Optimal Performance
- How is a Tube Furnace utilized in the color modification process of beryl? Master Deep Blue Aquamarine Transformation
- What chemical role does a tubular furnace play during the carbonization of Si@Sn@C? Unlock Advanced Material Synthesis
- Why are inert gases used in a High Temperature Tube Furnace? Prevent Oxidation and Ensure Process Precision



















