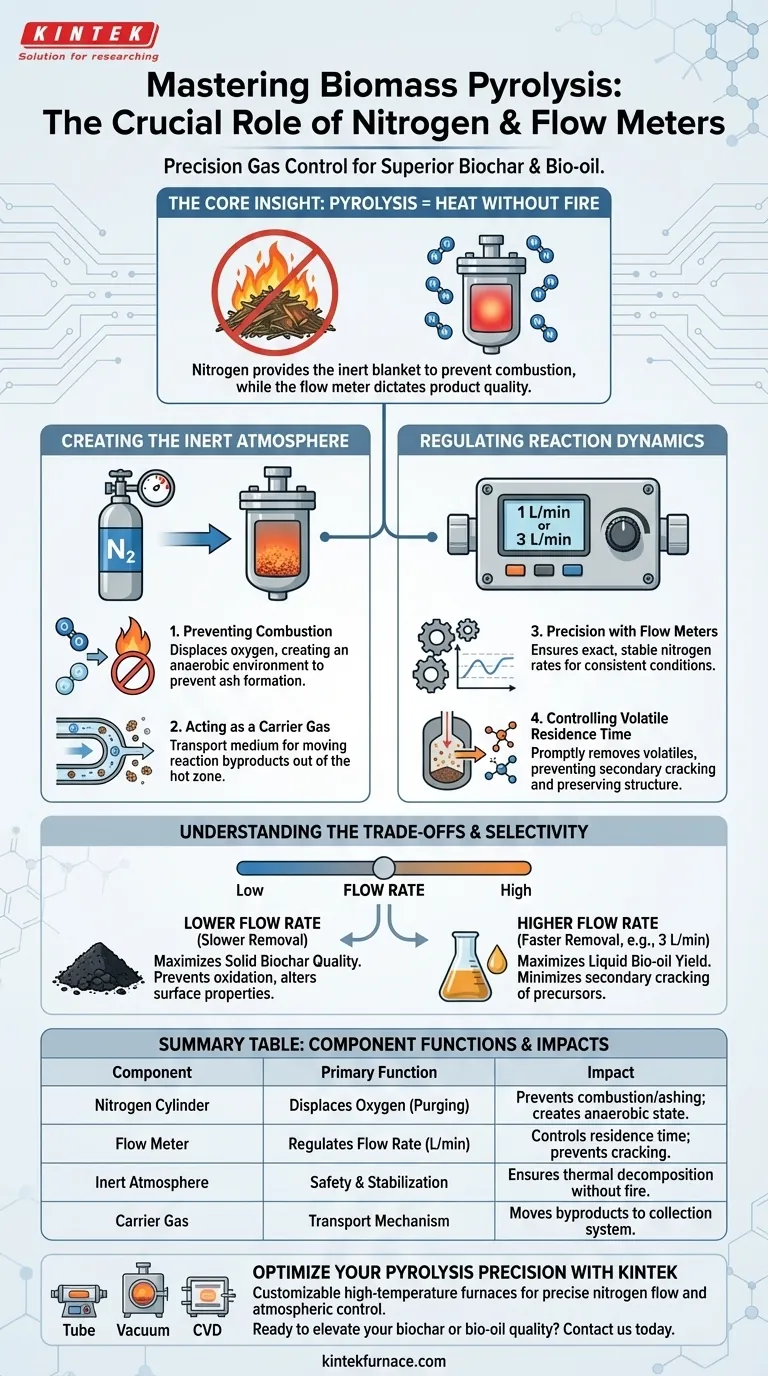
Nitrogen cylinders and flow meters function as the critical safety and process control mechanisms within a pyrolysis system. Together, they create and maintain the specific oxygen-free conditions required to thermally decompose biomass without burning it, while also managing the movement of gases to determine the final product quality.
The Core Insight Pyrolysis is distinct from burning; it requires heat without fire. Nitrogen provides the necessary inert blanket to prevent combustion, while the flow meter dictates how quickly volatile gases are swept away from the heat, directly influencing whether you produce high-quality biochar or maximize liquid bio-oil yields.

Creating the Inert Atmosphere
Preventing Combustion
The primary function of the nitrogen cylinder is to supply high-purity inert gas.
Because pyrolysis operates at high temperatures, the presence of even small amounts of oxygen would cause the biomass to ignite and turn into ash.
Nitrogen continuously purges air from the furnace, creating an anaerobic (oxygen-free) environment that allows thermal decomposition to occur safely.
Acting as a Carrier Gas
Beyond safety, nitrogen serves as a transport medium within the reactor.
It acts as a carrier gas, physically moving through the system to facilitate the chemical process.
This continuous flow is essential for moving reaction byproducts out of the hot zone.
Regulating Reaction Dynamics
Precision with Flow Meters
A flow meter is necessary because the volume of nitrogen must be exact, not estimated.
It allows operators to set specific rates, such as 1 L/min or 3 L/min, ensuring the environment remains stable throughout the experiment.
Without this precise regulation, the atmospheric conditions inside the furnace could fluctuate, leading to inconsistent results.
Controlling Volatile Residence Time
The flow meter directly impacts how long volatile gases remain inside the heated reaction zone.
By promptly carrying these volatiles away, the system prevents secondary cracking reactions.
If volatiles remain in the heat too long, they break down further; removing them quickly preserves their structure, which is critical for specific product yields.
Understanding the Trade-offs
The Balance of Flow Rates
Setting the flow meter is a balancing act that alters your chemical outcomes.
A flow rate that is too low may fail to purge oxygen completely or allow volatiles to re-condense on the biochar, altering its surface properties.
Conversely, a flow rate that is too high might sweep gases away too quickly, potentially affecting the heat transfer efficiency or the concentration of the collected products.
Product Selectivity
The specific setting you choose on the flow meter shifts the production balance between solid and liquid.
As noted in technical applications, adjusting the flow (e.g., to 3 L/min) to remove volatiles faster tends to favor the production of liquid bio-oil.
Slower removal rates or different configurations are often prioritized when the goal is maximizing the quality of solid biochar.
Making the Right Choice for Your Goal
To maximize the efficiency of your pyrolysis process, you must tune your nitrogen flow to your specific production targets.
- If your primary focus is Biochar Quality: Ensure the flow rate is sufficient to maintain a strictly oxygen-free environment to prevent oxidation of the carbon structure.
- If your primary focus is Bio-oil Yield: Increase the nitrogen flow rate to rapidly sweep volatiles from the reaction zone, minimizing secondary cracking that destroys liquid precursors.
Precision in gas control is the difference between burning biomass and refining it.
Summary Table:
| Component | Primary Function | Impact on Pyrolysis Outcome |
|---|---|---|
| Nitrogen Cylinder | Displaces Oxygen (Purging) | Prevents combustion/ashing; creates anaerobic state |
| Flow Meter | Regulates Flow Rate (L/min) | Controls residence time of volatiles; prevents cracking |
| Inert Atmosphere | Safety & Stabilization | Ensures thermal decomposition without fire |
| Carrier Gas | Transport Mechanism | Moves reaction byproducts to the collection system |
Optimize Your Pyrolysis Precision with KINTEK
Don't let inconsistent gas control compromise your research or production yields. KINTEK provides high-performance laboratory solutions backed by expert R&D and manufacturing. Whether you require Tube, Vacuum, or CVD systems, our high-temperature furnaces are fully customizable to meet your specific nitrogen flow and atmospheric requirements.
Ready to elevate your biochar or bio-oil quality? Contact us today to discuss how our specialized equipment can bring unparalleled control and safety to your biomass thermal decomposition projects.
Visual Guide
References
- S. S. Ibrahim, Badr A. Mohamed. Catalyzed biochar from date palm waste for ammonium removal: potential application in poultry farms for ammonia mitigation. DOI: 10.1007/s43621-025-00817-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does a constant temperature and humidity curing chamber contribute to GCCM hydration? Optimize Material Strength
- What are the advantages of using a precision vacuum drying oven? Master Ceramic Powder Treatment with KINTEK
- What is the purpose of using a laboratory blast drying oven at 107°C for 17 hours for reforming catalysts?
- Why is a constant temperature drying oven set to 60°C for 24 hours? Optimizing Sr4Al6O12SO4 Powder Quality
- Why is a standard constant temperature and humidity curing box used for magnesium slag mortar? Key Pre-treatment Facts
- What is the primary function of a laboratory blast drying oven? Mastering Coconut Husk Biochar Preparation
- Why is a graphite furnace better than a flame in AAS? Unlock Trace-Level Detection for Your Lab
- How does a vacuum drying oven contribute to the preparation of Na3(VO1-x)2(PO4)2F1+2x? Ensure High-Purity Synthesis



















