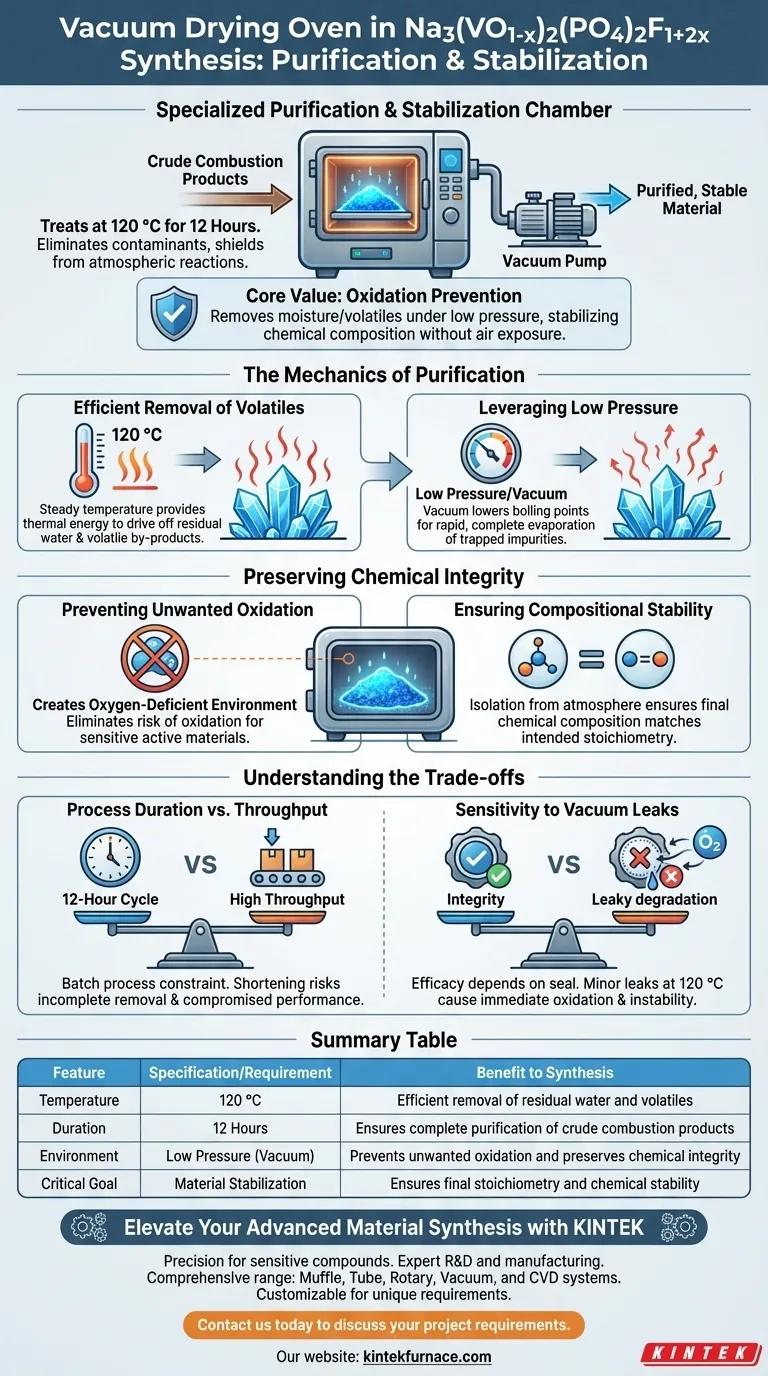
In the synthesis of Na3(VO1-x)2(PO4)2F1+2x, the vacuum drying oven functions as a specialized purification and stabilization chamber. Specifically, it treats crude combustion products at 120 °C for 12 hours to eliminate contaminants while shielding the material from atmospheric reactions.
The core value of vacuum drying in this context is oxidation prevention. By removing moisture and volatiles in a low-pressure environment, the process stabilizes the material's chemical composition without exposing reactive components to air.

The Mechanics of Purification
Efficient Removal of Volatiles
The primary operational goal of the vacuum drying oven is the removal of impurities from the crude combustion products.
By maintaining a steady temperature of 120 °C, the oven provides sufficient thermal energy to drive off residual water and volatile by-products.
Leveraging Low Pressure
The vacuum environment significantly lowers the boiling point of liquids and volatiles trapped within the material.
This allows for the rapid and complete evaporation of these impurities, which might otherwise remain trapped under standard atmospheric pressure.
Preserving Chemical Integrity
Preventing Unwanted Oxidation
The most critical contribution of the vacuum oven is the creation of an oxygen-deficient environment.
Because the active materials in Na3(VO1-x)2(PO4)2F1+2x are sensitive, exposure to air at elevated temperatures could lead to unwanted oxidation.
Operating under vacuum eliminates this risk, ensuring the material retains its intended oxidation state.
Ensuring Compositional Stability
The final stability of the product relies heavily on this isolation from the atmosphere during the drying phase.
By preventing side reactions with oxygen, the oven ensures that the final chemical composition matches the intended stoichiometry of the synthesis.
Understanding the Trade-offs
Process Duration vs. Throughput
While vacuum drying ensures high purity, it is a batch process that introduces a time constraint—specifically the 12-hour treatment cycle.
Skipping or shortening this duration to increase throughput can result in incomplete removal of volatiles, compromising the material's performance.
Sensitivity to Vacuum Leaks
The efficacy of this method is entirely dependent on the integrity of the vacuum seal.
Even a minor leak during the 120 °C heating phase allows oxygen to enter, which can immediately degrade the active material through oxidation, rendering the batch unstable.
Making the Right Choice for Your Goal
To maximize the quality of your Na3(VO1-x)2(PO4)2F1+2x synthesis, consider the following priorities:
- If your primary focus is Chemical Purity: Ensure the vacuum system is capable of maintaining a deep, consistent low-pressure environment throughout the entire 12-hour cycle to prevent any oxidative degradation.
- If your primary focus is Process Efficiency: strictly adhere to the 120 °C temperature set point; going lower may fail to remove volatiles, while going higher without vacuum validation risks thermal damage.
Precision in the drying phase is the final gatekeeper for material stability.
Summary Table:
| Feature | Specification/Requirement | Benefit to Synthesis |
|---|---|---|
| Temperature | 120 °C | Efficient removal of residual water and volatiles |
| Duration | 12 Hours | Ensures complete purification of crude combustion products |
| Environment | Low Pressure (Vacuum) | Prevents unwanted oxidation and preserves chemical integrity |
| Critical Goal | Material Stabilization | Ensures final stoichiometry and chemical stability |
Elevate Your Advanced Material Synthesis with KINTEK
Precision is non-negotiable when synthesizing sensitive compounds like Na3(VO1-x)2(PO4)2F1+2x. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your laboratory's unique high-temperature and atmospheric requirements.
Our vacuum ovens provide the stable, oxygen-free environments necessary to prevent oxidation and ensure the chemical integrity of your active materials. Contact us today to discuss your project requirements and discover how our specialized lab solutions can enhance your research outcomes.
Visual Guide
References
- Oskar Grabowski, A. Czerwiński. Solution-combustion synthesis of Na3(VO1-x)2(PO4)2F1+2x as a positive electrode material for sodium-ion batteries. DOI: 10.1038/s44172-025-00471-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What are the technical advantages of using a pyrolysis furnace vs. an incinerator? Recover Value from Composites
- What is the use of a laboratory furnace? Unlock Precise Material Transformation
- What are the advantages of using an acid oxidation bath? Accelerate Lignin Fiber Stabilization from Hours to Minutes
- What is made in a dental lab? Discover the Custom Prosthetics for Your Smile
- What role do low-temperature carbonization furnaces play in carbon fiber manufacture? Build a Strong Structural Foundation
- What is the role of high-pressure inert gases in the HPB process? Mastering CZT Crystal Stoichiometry
- Why is a precision furnace required after TiO2-alpha-Ga2O3 synthesis? Master Phase Transformation & Interface Bonding
- What are the key characteristics of furnaces used in 3D printing sintering? Achieve Precision Sintering for High-Quality Parts



















