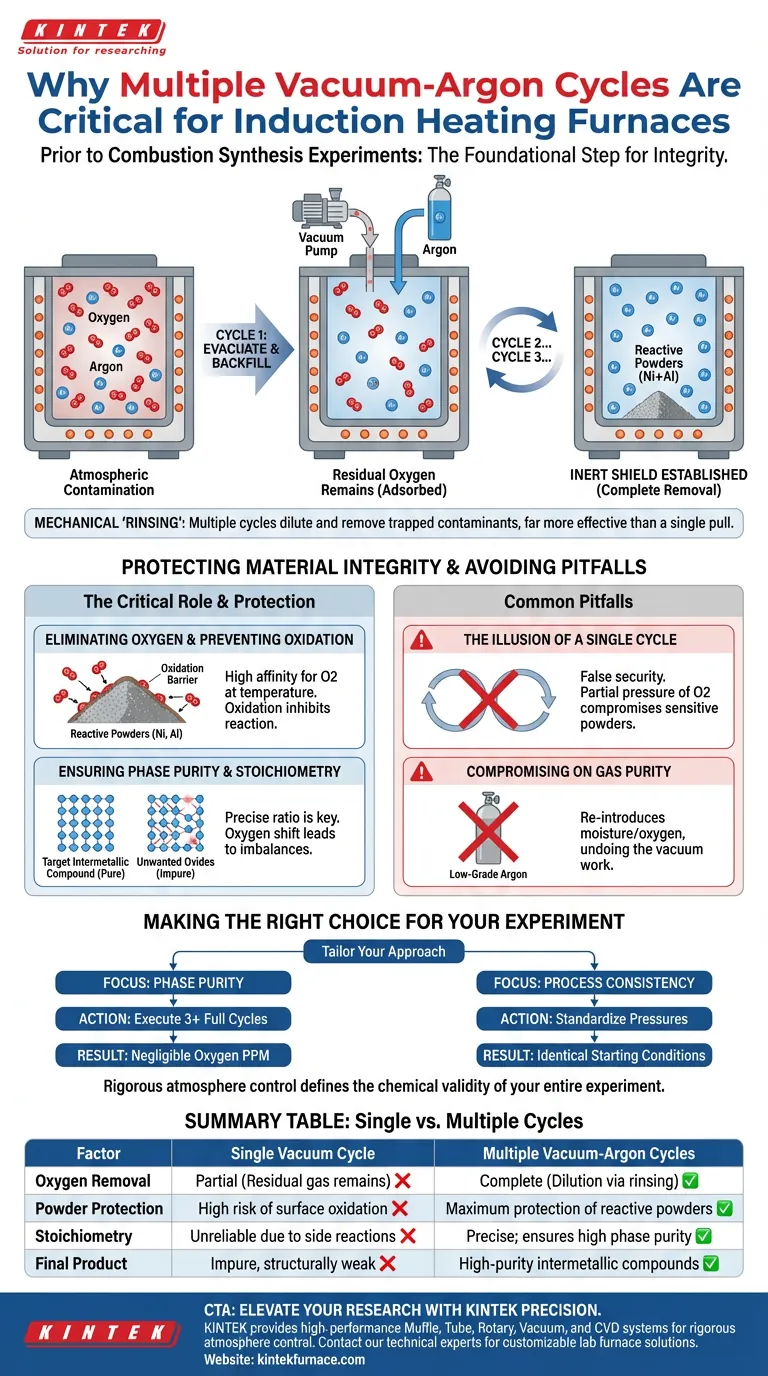
Multiple vacuum-argon cycles represent the foundational step for ensuring the integrity of combustion synthesis experiments inside an induction heating furnace. This repetitive process is required to fully purge atmospheric oxygen from the furnace chamber and replace it with a protective, inert argon atmosphere, thereby preventing the degradation of reactive metal powders.
A single evacuation is rarely sufficient to remove all contaminants; performing multiple cycles ensures the complete removal of oxygen, preserving the correct chemical stoichiometry and phase purity of the final intermetallic compound.
The Critical Role of Atmosphere Control
Eliminating Residual Oxygen
The primary objective of these cycles is the total evacuation of oxygen. High-vacuum pumps are effective, but a single pull often leaves residual gas molecules adsorbed to the chamber walls or trapped within the powder bed.
By repeatedly flushing the chamber with argon and re-evacuating it, you dilute and remove these remaining contaminants. This mechanical "rinsing" of the atmosphere is far more effective than a single, prolonged vacuum stage.
Establishing an Inert Shield
Once the oxygen is removed, the final stage involves backfilling the chamber with high-purity argon. This creates a non-reactive environment that envelopes the sample.
This inert shield is necessary to facilitate the high temperatures required for induction heating without triggering unwanted side reactions with the surrounding air.
Protecting Material Integrity
Preventing Powder Oxidation
Combustion synthesis often utilizes highly reactive raw materials, such as nickel and aluminum powders. These metals have a high affinity for oxygen, especially as temperatures rise.
Without a strictly inert atmosphere, these powders will oxidize rapidly before the synthesis reaction can occur. This oxidation creates a barrier between particles, inhibiting the desired reaction mechanism.
Ensuring Phase Purity and Stoichiometry
The success of the experiment depends on a precise ratio of reactants, known as chemical stoichiometry. If oxygen consumes a portion of the aluminum or nickel, the ratio of the remaining available metal shifts.
This imbalance leads to the formation of unwanted oxides rather than the target nickel-aluminum intermetallic compound. Multiple cycles ensure that the final product maintains high phase purity and the correct chemical composition.
Common Pitfalls to Avoid
The Illusion of a Single Cycle
A common error is assuming that reaching a high vacuum level once is sufficient. Even at high vacuum, the partial pressure of oxygen can remain high enough to compromise sensitive nanometric or micrometric powders.
Skipping the "cycle" aspect creates a false sense of security, often resulting in samples that are structurally weak or chemically impure.
Compromising on Gas Purity
The effectiveness of this process is entirely dependent on the quality of the argon used during the backfill stages.
Using low-grade argon introduces moisture or trace oxygen back into the system, effectively undoing the work of the vacuum pump and contaminating the synthesis.
Making the Right Choice for Your Experiment
To maximize the success of your nickel-aluminum combustion synthesis, tailor your approach based on your specific requirements:
- If your primary focus is Phase Purity: Execute at least three full vacuum-argon cycles to mathematically minimize oxygen parts-per-million to negligible levels.
- If your primary focus is Process Consistency: Standardize the specific vacuum pressure and argon backfill pressure for every cycle to ensure identical starting conditions for every run.
Rigorous atmosphere control is not just a preparation step; it is the variable that defines the chemical validity of your entire experiment.
Summary Table:
| Factor | Single Vacuum Cycle | Multiple Vacuum-Argon Cycles |
|---|---|---|
| Oxygen Removal | Partial (residual gas remains) | Complete (dilution via mechanical rinsing) |
| Powder Protection | High risk of surface oxidation | Maximum protection of reactive powders |
| Stoichiometry | Unreliable due to side reactions | Precise; ensures high phase purity |
| Final Product | Impure, structurally weak | High-purity intermetallic compounds |
Elevate Your Research with KINTEK Precision
Don't let atmospheric contamination compromise your results. KINTEK provides high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, specifically engineered for rigorous atmosphere control. Backed by expert R&D and manufacturing, our lab high-temp furnaces are fully customizable to meet the exacting demands of combustion synthesis and material science.
Ready to ensure perfect stoichiometry and phase purity?
Contact our technical experts today to find the ideal furnace solution for your unique laboratory needs.
Visual Guide
References
- Gülizar Sarıyer, H. Erdem Çamurlu. Production and Characterization of Ni0.50 Al0.50 and Ni0.55 Al0.45 Powders by Volume Combustion Synthesis. DOI: 10.17776/csj.1280582
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- Why is a non-consumable vacuum arc melting furnace used for AlCrTiVNbx alloys? Ensure Purity & Homogeneity
- In what ways does induction heating promote resource efficiency? Achieve Precision, Speed, and Sustainability
- What role does a medium-frequency induction vacuum furnace play in melting S30403? Achieve Pure Alloy Integrity
- How does a vacuum induction melting furnace (VIM furnace) work? Achieve Ultra-Pure Metals with Precision Melting
- What are the key steps in the vacuum induction melting process? Achieve High-Purity Metal Alloys for Demanding Applications
- Why are induction furnaces popular for alloy manufacturing? Achieve Superior Alloy Homogeneity and Efficiency
- What is the historical background of induction furnace development? From Faraday to Modern Metallurgy
- Why are induction furnaces suitable for investment casting? Precision Melting for Complex Casts



















