Graphite molds are the standard choice for the vacuum hot press sintering of aluminum-based composites due to their exceptional combination of thermal stability, chemical inertness, and mechanical strength. They withstand elevated temperatures while effectively transferring hydraulic pressure to the powder body, ensuring dimensional accuracy and high forming quality without reacting with the aluminum alloy.
The core value of graphite in this process is its ability to act simultaneously as a heat conductor, a pressure vessel, and a non-reactive shield. It enables the densification of reactive aluminum powders into precise shapes under extreme conditions where other mold materials would fail or contaminate the product.

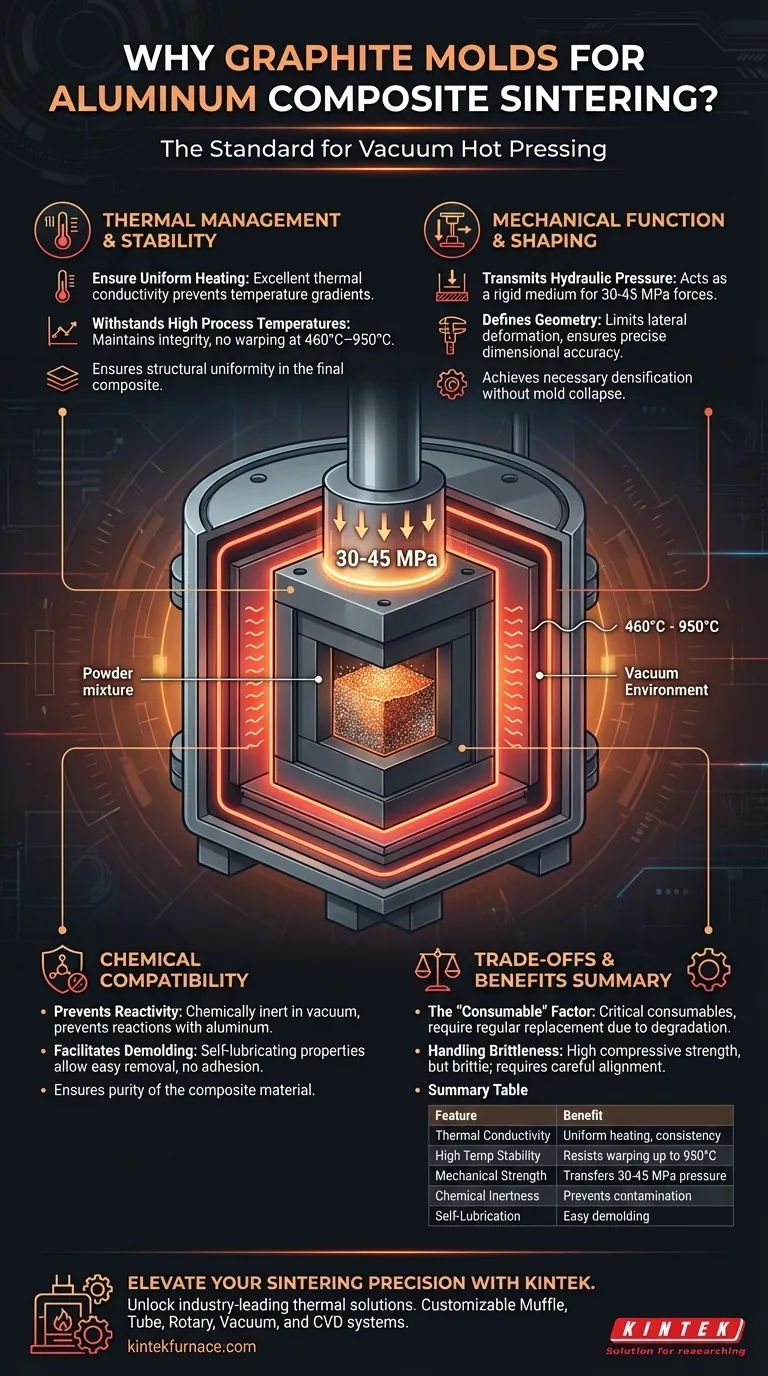
Thermal Management and Stability
Ensuring Uniform Heating
Graphite possesses excellent thermal conductivity. This property is essential for transferring heat from the furnace elements to the internal powder body efficiently.
By ensuring uniform heat distribution, graphite prevents temperature gradients within the mold. This guarantees that the composite material sinters evenly, resulting in structural uniformity throughout the final part.
Withstanding Process Temperatures
Vacuum hot pressing often requires temperatures ranging from roughly 460°C to as high as 950°C.
Graphite exhibits superior thermal stability in these ranges. Unlike many metals that might soften or warp, graphite maintains its structural integrity and does not deform under high heat, ensuring the sample geometry remains consistent.
Mechanical Function and Shaping
Transmitting Hydraulic Pressure
The sintering process relies on high pressure—typically between 30 MPa and 45 MPa—to densify the powder.
Graphite has sufficient high-temperature mechanical strength to withstand these axial loads. It acts as a rigid medium to transfer the force from the press directly to the powder, achieving the necessary density without the mold collapsing.
Defining Geometry
The mold serves as the primary containment vessel for mixed powders or stacked foils.
It limits lateral deformation during the pressing phase. This containment ensures the final "ingot" or component achieves precise dimensional accuracy and forms the correct shape.
Chemical Compatibility
Preventing Reactivity
Aluminum and its alloys (such as Aluminum-Tin) are highly reactive, particularly in molten or semi-solid states.
Graphite is chemically inert in a vacuum environment. This prevents severe chemical reactions between the mold and the aluminum matrix, ensuring the chemical composition of the composite remains pure and unaltered.
Facilitating Demolding
Graphite naturally possesses self-lubricating properties and resists adhesion to metal matrices.
Because the aluminum does not stick or chemically bond to the graphite wall, the sintered sample can be easily removed (demolded) after the process. This reduces the risk of damaging the final part during extraction.
Understanding the Trade-offs
The "Consumable" Factor
While graphite is robust, these molds are generally treated as critical consumables.
Repeated exposure to thermal cycling and high mechanical pressure will eventually degrade the mold. They must be inspected and replaced regularly to ensure they continue to produce dimensionally accurate parts.
Handling Brittleness
Graphite has high compressive strength, but it is inherently brittle compared to steel tools.
It requires careful alignment and handling within the press. Uneven loading or shear forces can cause the mold to fracture, unlike ductile metal molds which might deform before breaking.
Making the Right Choice for Your Goal
When designing a sintering process for aluminum composites, graphite is selected to balance several competing requirements.
- If your primary focus is Dimensional Precision: Rely on graphite’s high-temperature stiffness to maintain exact tolerances under pressures up to 45 MPa.
- If your primary focus is Material Purity: Leverage graphite's chemical inertness to prevent surface contamination or alloying between the mold and the aluminum.
- If your primary focus is Process Efficiency: Utilize graphite’s thermal conductivity to reduce cycle times through faster, more uniform heating and cooling.
Graphite effectively bridges the gap between a structural container and a thermal instrument, making it indispensable for high-quality powder densification.
Summary Table:
| Feature | Benefit for Aluminum Sintering |
|---|---|
| Thermal Conductivity | Ensures uniform heating and structural consistency in the composite. |
| High Temperature Stability | Resists warping and maintains geometry at temperatures up to 950°C. |
| Mechanical Strength | Transfers 30-45 MPa of hydraulic pressure without mold collapse. |
| Chemical Inertness | Prevents contamination or reactions between the mold and reactive aluminum. |
| Self-Lubrication | Facilitates easy demolding and reduces damage to the final sintered part. |
Elevate Your Sintering Precision with KINTEK
Unlock the full potential of your material science projects with industry-leading thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other laboratory high-temperature furnaces—all fully customizable to meet your unique sintering requirements.
Whether you are processing aluminum-based composites or advanced ceramics, our equipment ensures the thermal stability and pressure control your research demands. Contact us today to discuss your custom furnace needs and see how our expertise can drive your innovation forward.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What is the function of applying axial pressure during hot press sintering? Achieve High-Density Metal Composites
- What are the primary applications of vacuum hot press furnaces? Achieve Superior Material Density and Purity
- What is the role of sacrificial inserts in the Spark Plasma Sintering (SPS) process? Master Complex Geometry Design
- What are the advantages of using WC-Co anvils in UHP-SPS? Unlock Extreme Sintering Pressures and Material Density
- How does the heating mechanism of Spark Plasma Sintering (SPS) function? Enhance TiC/SiC Composite Fabrication
- What is hot pressing and what does it involve? Unlock Superior Material Density and Strength
- What other types of furnaces are related to hot pressing? Explore Key Thermal Processing Technologies
- Why is it necessary to maintain a high vacuum environment during the SPS of SiC? Key to High-Density Ceramics



















