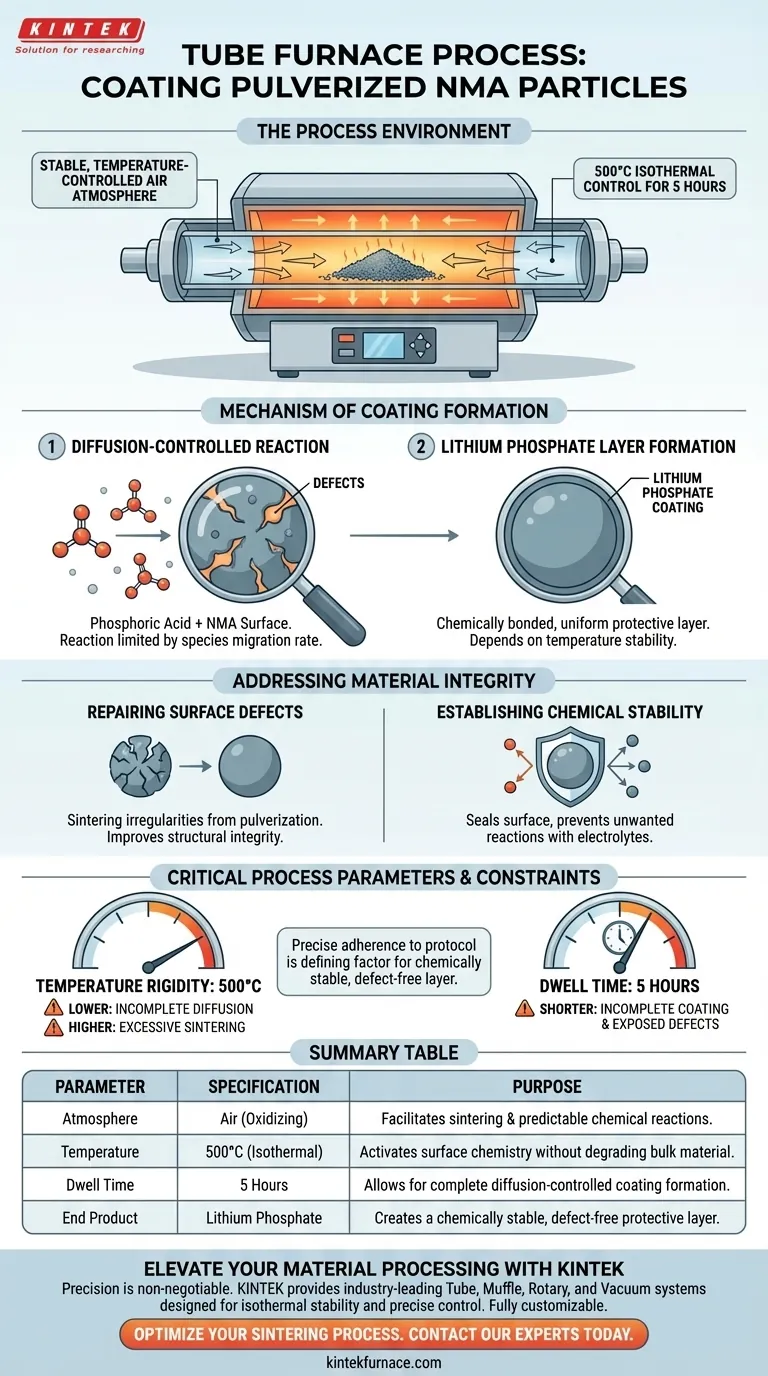
A tube furnace establishes a highly stable, temperature-controlled air atmosphere specifically designed for the sintering of pulverized NMA particles. During this coating stage, the furnace maintains a constant temperature of 500°C for a continuous period of 5 hours. This specific thermal environment is engineered to support chemical reactions that are sensitive to both temperature stability and atmospheric composition.
The controlled thermal environment facilitates a critical diffusion reaction between phosphoric acid and the particle surface, transforming surface defects into a uniform, chemically stable lithium phosphate protective layer.

The Role of the Thermal Environment
Precise Temperature Regulation
The primary function of the tube furnace in this context is isothermal control.
The system must hold the pulverized NMA (nickel-manganese-aluminum) particles at exactly 500°C.
This constant heat input provides the necessary energy to activate the surface chemistry without degrading the bulk material.
The Air Atmosphere
Unlike processes requiring inert gases or vacuums, this coating stage operates within an air atmosphere.
The presence of air at elevated temperatures creates the oxidizing environment necessary for the specific sintering reactions to occur.
It ensures the reactants behave predictably during the prolonged heating phase.
Mechanism of Coating Formation
Diffusion-Controlled Reaction
The 500°C environment drives a reaction between the phosphoric acid introduced to the system and the surfaces of the NMA particles.
This is a diffusion-controlled process.
This means the reaction rate is limited by how quickly the chemical species can migrate through the developing interface, necessitating the long 5-hour duration.
Creating the Lithium Phosphate Layer
The outcome of this thermal treatment is the formation of a lithium phosphate coating.
This layer is not merely a deposit; it is chemically bonded to the particle.
The uniformity of this layer is directly dependent on the stability of the furnace temperature.
Addressing Material Integrity
Repairing Surface Defects
Pulverization is a mechanical process that naturally damages the surface of NMA particles.
The tube furnace treatment acts as a restorative phase, sintering these irregularities.
By smoothing out these defects, the process improves the structural integrity of the individual particles.
Establishing Chemical Stability
The coating does more than just repair physical damage; it seals the surface.
The lithium phosphate layer acts as a barrier, preventing unwanted reactions between the NMA core and electrolytes in a battery system.
This step is essential for converting raw, pulverized powder into a usable, long-lasting material.
Understanding Process Constraints
The Cost of Time
The process requires a significant dwell time of 5 hours.
Because the reaction is diffusion-controlled, rushing this step is a common pitfall.
Reducing the time below the specified duration will likely result in an incomplete coating and exposed surface defects.
Temperature Rigidity
The target temperature of 500°C is a critical parameter, not a guideline.
Deviating from this setpoint risks altering the reaction kinetics.
Lower temperatures may fail to drive the diffusion, while higher temperatures could lead to excessive sintering or particle agglomeration.
Making the Right Choice for Your Goal
To ensure the highest quality coating on pulverized NMA particles, prioritize your process parameters based on the desired outcome:
- If your primary focus is coating uniformity: rigorous temperature control at 500°C is required to ensure the diffusion reaction occurs at a constant rate across the entire batch.
- If your primary focus is surface repair: strictly adhere to the 5-hour duration to allow sufficient time for the diffusion mechanism to heal physical defects caused by pulverization.
Precise adherence to this thermal protocol is the defining factor in achieving a chemically stable, defect-free protective layer.
Summary Table:
| Parameter | Specification | Purpose in Coating Stage |
|---|---|---|
| Atmosphere | Air (Oxidizing) | Facilitates sintering and predictable chemical reactions. |
| Temperature | 500°C (Isothermal) | Activates surface chemistry without degrading bulk material. |
| Dwell Time | 5 Hours | Allows for complete diffusion-controlled coating formation. |
| End Product | Lithium Phosphate | Creates a chemically stable, defect-free protective layer. |
Elevate Your Material Processing with KINTEK
Precision is non-negotiable when dealing with diffusion-controlled reactions like NMA particle coating. KINTEK provides industry-leading Tube, Muffle, Rotary, and Vacuum systems designed for isothermal stability and precise atmospheric control. Backed by expert R&D and manufacturing, our high-temperature lab furnaces are fully customizable to meet your unique chemical and thermal requirements.
Ready to optimize your sintering process? Contact our experts today to find the perfect thermal solution for your lab.
Visual Guide
References
- Alexis Luglio, Ryan Brow. Maximizing calendering effects through the mechanical pulverization of Co-free nickel-rich cathodes in lithium-ion cells. DOI: 10.1557/s43577-025-00936-5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is thermal uniformity important in a drop tube furnace? Ensure Reliable Results and Consistent Processes
- How does a high-precision tube furnace using an argon atmosphere facilitate the adjustment of copper foil surface roughness?
- How does the working temperature range affect the choice of a vertical tube furnace? Optimize Your Lab's Performance and Budget
- What is the primary function of a tube furnace in materials science and engineering? Unlock Precise High-Temperature Processing
- What are the technical advantages of using a high-temperature tube furnace? Precision Thermal Oxidation Explained
- What are the technical advantages of using a vacuum tube furnace for S53P4-NO2 glass? Achieve 100% Amorphous Results
- What is a vertical tube furnace used for in semiconductor manufacturing? Essential for High-Precision Thermal Processing
- How does the diversification of vacuum tube furnaces impact the market? Unlock Specialized Solutions for Advanced Materials



















