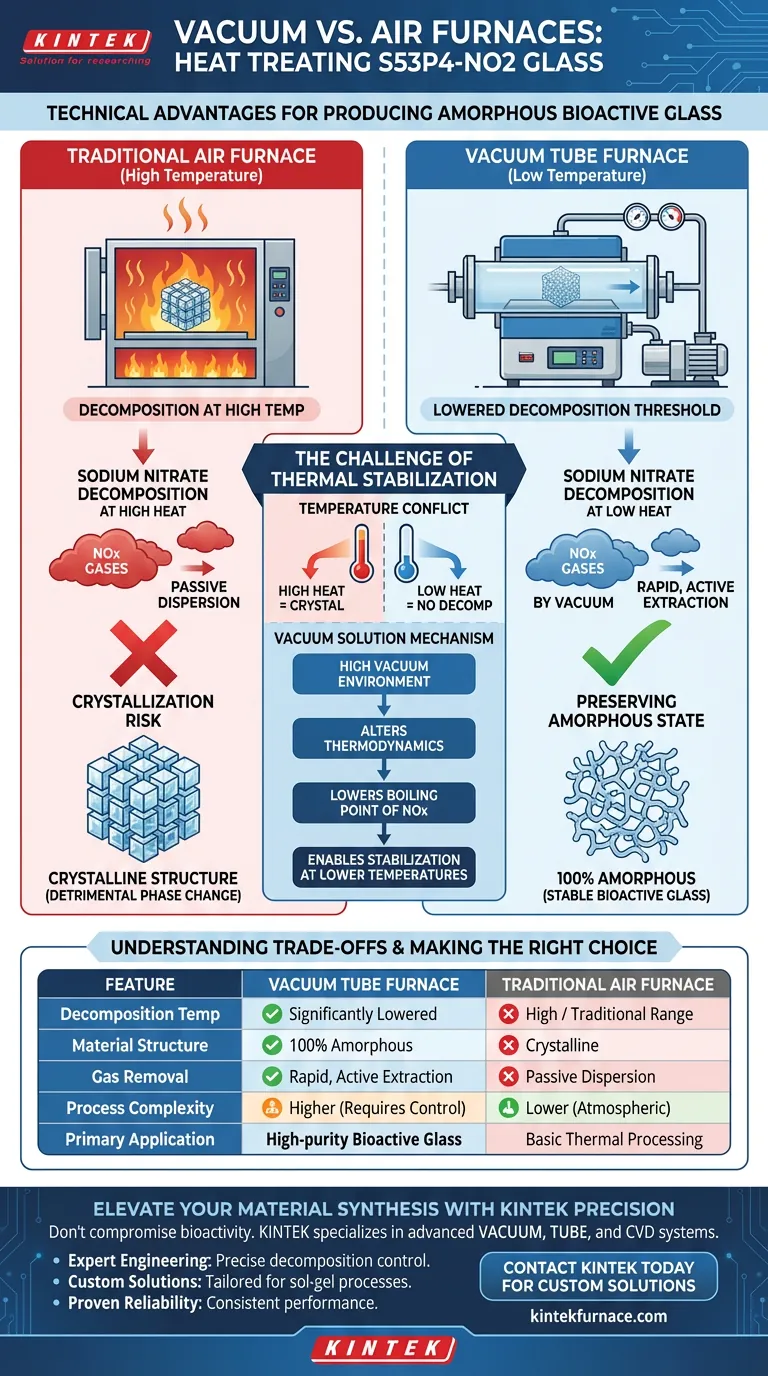
The primary technical advantage of using a vacuum tube furnace for the heat treatment of S53P4-NO2 glass is the ability to produce a completely amorphous material by preventing crystallization. By utilizing a high-vacuum environment, the furnace significantly lowers the decomposition temperature of precursor salts like sodium nitrate, allowing nitrogen oxide gases to be removed rapidly without subjecting the glass to the high temperatures that typically cause unwanted structural changes in standard air furnaces.
Core Takeaway Traditional thermal treatment often forces a compromise between precursor decomposition and material structure. A vacuum tube furnace resolves this by altering the thermodynamics of the process, enabling the stabilization of sodium-containing sol-gel bioactive glass at lower temperatures to ensure it remains 100% amorphous.
The Challenge of Thermal Stabilization
The Temperature Conflict
In the production of S53P4-NO2 glass, the stabilization process requires the decomposition of precursor salts, specifically sodium nitrate.
In a traditional air furnace, this decomposition requires high temperatures.
The Crystallization Risk
The heat required to break down these salts in an air atmosphere creates a critical problem for the material's structure.
At these elevated temperatures, the glass matrix tends to crystallize. This phase change is detrimental when the goal is to produce a completely amorphous bioactive glass.
Mechanisms of the Vacuum Solution
Lowering Decomposition Thresholds
The introduction of a high-vacuum environment fundamentally changes the decomposition kinetics of the precursor salts.
Under vacuum, the temperature required to decompose sodium nitrate is significantly reduced. This allows the process to occur at a thermal range where the glass structure remains stable.
Rapid Gas Removal
As the salts decompose, they release nitrogen oxide gases.
The vacuum system does not just lower the boiling point; it actively and rapidly removes these gases from the reaction chamber. This efficient extraction prevents gas entrapment and facilitates the stabilization process.
Preserving the Amorphous State
The ultimate technical benefit is the preservation of the material's non-crystalline nature.
Because the vacuum allows for processing at lower temperatures, the glass avoids the thermal energy threshold that triggers crystallization. The result is a completely amorphous sodium-containing sol-gel bioactive glass.
Understanding the Trade-offs
Equipment Complexity
While the material benefits are clear, vacuum tube furnaces represent a more complex engineering solution than standard air furnaces.
Users must account for the maintenance of vacuum pumps and the integrity of seals.
Operational Overhead
Achieving high vacuum adds a layer of process control that is not present in atmospheric heating.
This requires precise monitoring of pressure levels alongside temperature profiles to ensure the decomposition benefits are fully realized.
Making the Right Choice for Your Goal
To determine if a vacuum tube furnace is required for your specific application, consider the following technical priorities:
- If your primary focus is material purity and bioactivity: You must use a vacuum furnace to ensure the glass remains completely amorphous and free of crystalline defects.
- If your primary focus is simplified processing: A traditional air furnace offers easier operation, but you must accept that crystallization will occur due to the higher temperatures required for salt decomposition.
The vacuum tube furnace is not merely an alternative heating method; it is a requisite tool for synthesizing amorphous S53P4-NO2 glass.
Summary Table:
| Feature | Vacuum Tube Furnace | Traditional Air Furnace |
|---|---|---|
| Decomposition Temperature | Significantly Lowered | High / Traditional Range |
| Material Structure | 100% Amorphous (No Crystallization) | Crystalline (Structural Change) |
| Gas Removal | Rapid, active extraction of NOx | Passive dispersion |
| Process Complexity | Higher (requires vacuum control) | Lower (atmospheric) |
| Primary Application | High-purity Bioactive Glass Synthesis | Basic thermal processing |
Elevate Your Material Synthesis with KINTEK Precision
Don't compromise the bioactivity of your S53P4-NO2 glass with unwanted crystallization. At KINTEK, we specialize in providing advanced Vacuum, Tube, and CVD systems designed to solve complex thermal challenges. Backed by expert R&D and world-class manufacturing, our lab high-temp furnaces are fully customizable to meet your unique research needs.
Why choose KINTEK?
- Expert Engineering: Specialized vacuum systems for precise decomposition control.
- Custom Solutions: Tailored furnace configurations for specific sol-gel glass processes.
- Proven Reliability: Built for consistent performance in high-stakes laboratory environments.
Ready to stabilize your bioactive materials at optimal temperatures? Contact us today to find your custom solution!
Visual Guide
References
- Jian Zheng, Julian R. Jones. Sol‐gel derived S53P4 bioactive glass. DOI: 10.1111/jace.70090
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the benefits of using a high-temperature vacuum furnace for the annealing of ZnSeO3 nanocrystals?
- What does a vacuum furnace do? Achieve Superior Material Processing in a Pure Environment
- How does a vacuum heat treatment furnace prevent contamination? Ensure Purity in High-Temperature Processes
- How does the ultra-low oxygen environment of vacuum sintering affect titanium composites? Unlock Advanced Phase Control
- What does the vacuum system of a vacuum furnace consist of? Essential Components for Clean Heat Processing



















