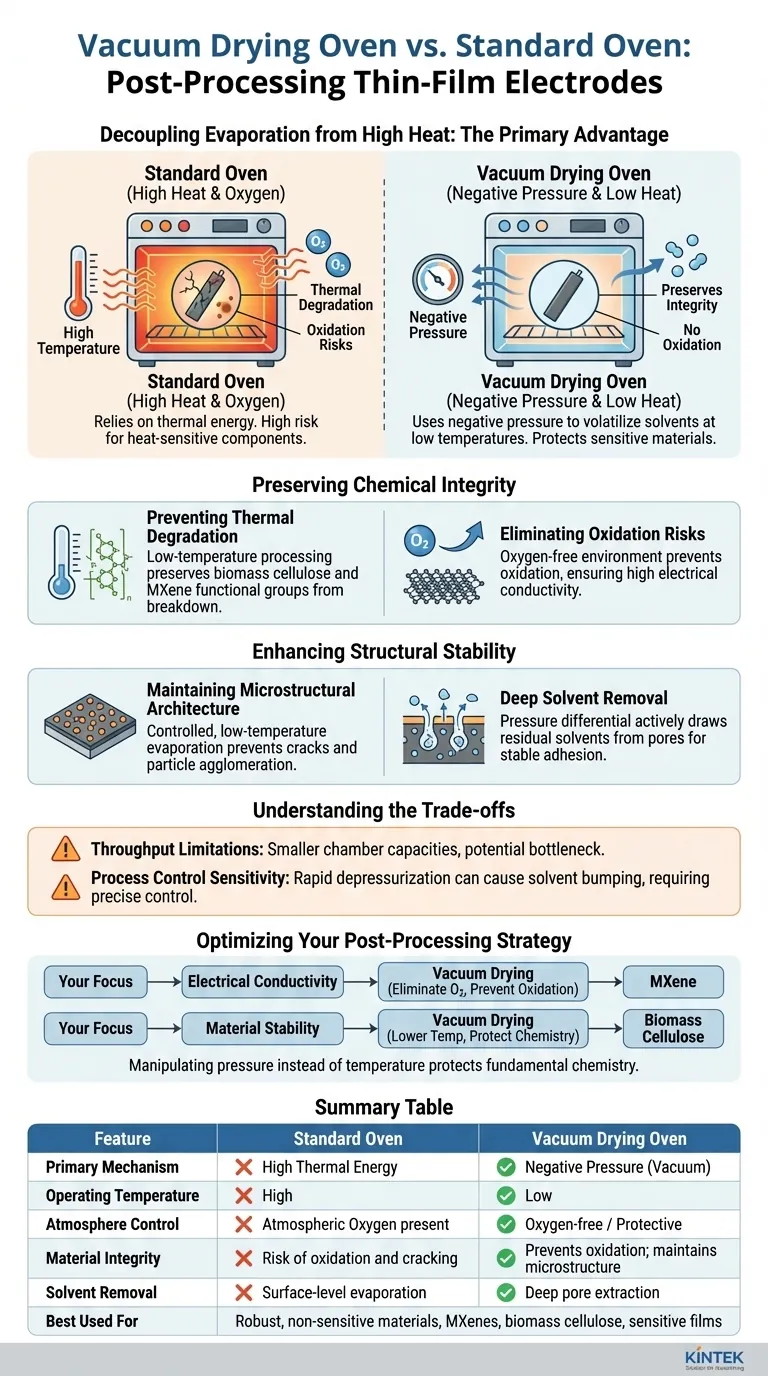
The primary technical advantage of a vacuum drying oven lies in its ability to decouple evaporation from high heat through the application of negative pressure. This allows for the rapid removal of moisture and residual solvents from thin-film electrodes without subjecting them to the damaging temperatures required by a standard oven.
By lowering the boiling point of solvents, vacuum drying enables efficient processing at reduced temperatures. This preserves the chemical stability of sensitive materials like MXenes and biomass cellulose, preventing oxidation and thermal degradation that would otherwise compromise the electrode's conductivity and structure.

Preserving Chemical Integrity
Preventing Thermal Degradation
Standard ovens rely on thermal energy to drive off solvents, which poses a risk to heat-sensitive components.
Vacuum drying uses negative pressure to volatilize solvents at significantly lower temperatures.
This is critical for electrodes containing biomass cellulose or specific MXene functional groups, as it prevents the breakdown of these materials that typically occurs in high-heat environments.
Eliminating Oxidation Risks
In a standard oven, the combination of heat and atmospheric oxygen accelerates oxidation.
The vacuum environment removes oxygen from the drying chamber, providing a protective atmosphere for reactive materials.
For MXene-based electrodes, this is essential to prevent oxidation, ensuring the material maintains its high electrical conductivity.
Enhancing Structural Stability
Maintaining Microstructural Architecture
Rapid, high-temperature evaporation can induce stress, leading to cracks or defects in thin films.
Vacuum drying facilitates a controlled, low-temperature evaporation process that preserves the structural integrity of the electrode.
This approach prevents the migration or agglomeration of active particles (such as platinum or metal halides), ensuring active sites remain evenly distributed.
Deep Solvent Removal
Thin-film electrodes often trap solvents within their porous structures.
The pressure differential in a vacuum oven actively draws residual solvents (like isopropanol or DMF) out of the material pores.
This ensures thorough drying and stable physical adhesion of active materials to the substrate, such as carbon cloth, without requiring harsh thermal treatment.
Understanding the Trade-offs
While vacuum drying offers superior preservation of material properties, it introduces operational complexities compared to standard ovens.
Throughput Limitations: Vacuum ovens generally have smaller chamber capacities than standard convection ovens, potentially creating a bottleneck for high-volume manufacturing.
Process Control Sensitivity: If the pressure is reduced too rapidly, solvents may boil violently (bumping). This can disrupt the uniform coating of the thin film or detach materials from the substrate, requiring precise control over the depressurization rate.
Optimizing Your Post-Processing Strategy
Choosing the right drying method depends heavily on the specific sensitivity of your electrode materials.
- If your primary focus is Electrical Conductivity: Prioritize vacuum drying to eliminate oxygen and prevent the oxidation of conductive materials like MXene.
- If your primary focus is Material Stability: Use vacuum drying to lower the processing temperature, protecting biomass cellulose and functional groups from thermal decomposition.
By manipulating pressure rather than temperature, you protect the fundamental chemistry of your electrode, ensuring the final device performs exactly as engineered.
Summary Table:
| Feature | Standard Oven | Vacuum Drying Oven |
|---|---|---|
| Primary Mechanism | High Thermal Energy | Negative Pressure (Vacuum) |
| Operating Temperature | High (High risk of degradation) | Low (Preserves heat-sensitive materials) |
| Atmosphere Control | Atmospheric Oxygen present | Oxygen-free / Protective |
| Material Integrity | Risk of oxidation and cracking | Prevents oxidation; maintains microstructure |
| Solvent Removal | Surface-level evaporation | Deep pore extraction (pressure differential) |
| Best Used For | Robust, non-sensitive materials | MXenes, biomass cellulose, sensitive films |
Elevate Your Electrode Performance with KINTEK
Don't let high heat compromise your research. KINTEK’s advanced vacuum drying solutions are engineered to protect the chemical integrity and structural architecture of your most sensitive materials. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your laboratory’s unique requirements.
Ensure maximum conductivity and stability for your thin-film electrodes—Contact our experts today to find your perfect high-temp furnace solution!
Visual Guide
References
- Lina Liu, Xuecheng Chen. Multilayered MXene/Pristine Carbon/Biomass Cellulose Film Electrode with Ultrahigh Volumetric Capacitance for Symmetric Flexible Supercapacitor. DOI: 10.1002/cmtd.202500036
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- How does the vacuum brazing process work? Achieve Clean, Strong Metal Joining Without Flux
- How does the thermal conductivity of graphite felt compare to graphite board at 1150 °C? A Guide to High-Temp Insulation
- How does a high-pressure gas quenching system maintain dimensional stability? Mastering Uniform Cooling Precision
- What are the maintenance requirements for a vacuum furnace when not in use? Protect Your Investment with Proper Storage
- What advantages does a vacuum drying oven offer over a standard oven for Fe3Al and CNTs? Protect Your Composites
- Why is a laboratory vacuum oven with nitrogen protection used for alumina dehydration? Ensure High-Purity Surfaces
- Why is a vacuum deposition system required for BL-MoS2 doping? Achieve Nanogram-Level Precision & Purity
- How do vacuum furnaces ensure precise heat treatment results? Master Control for Superior Material Properties



















