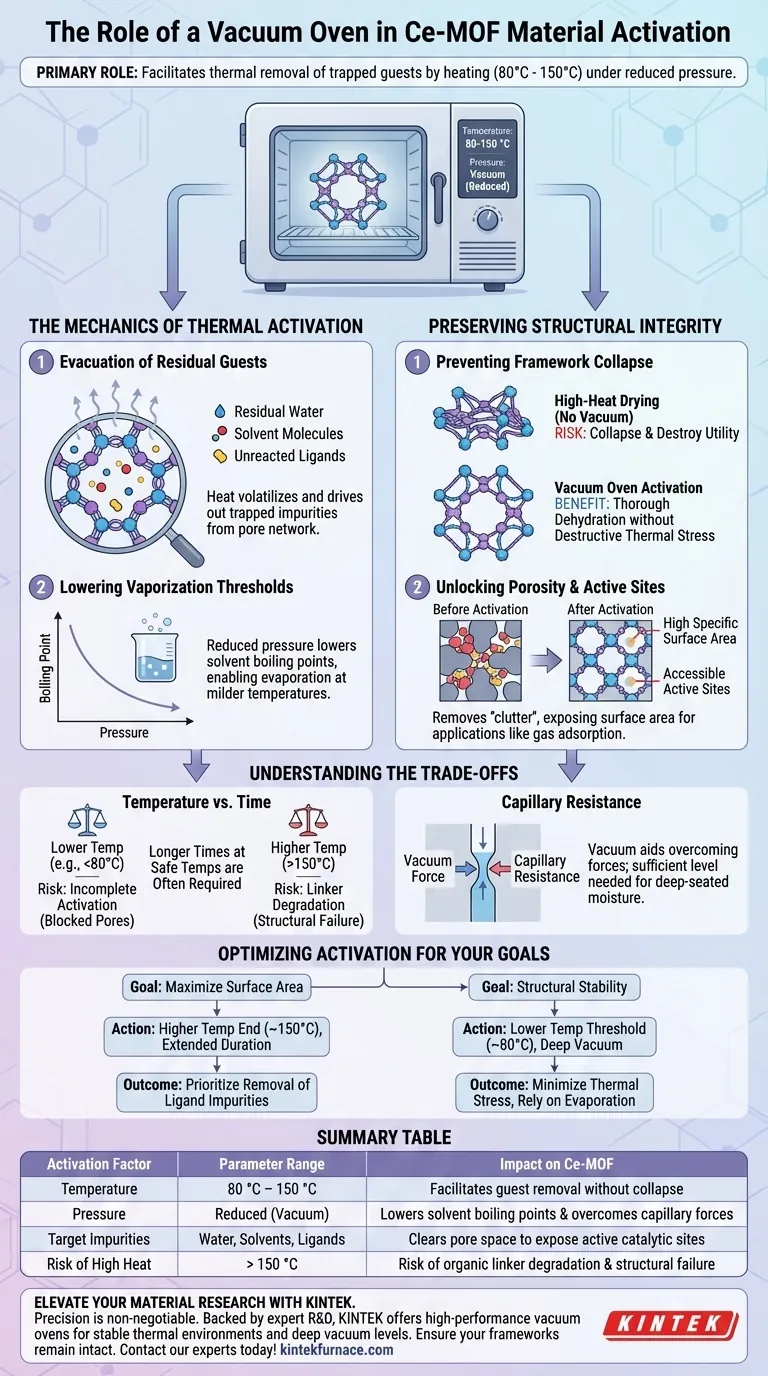
The primary role of a vacuum oven in Ce-MOF activation is to facilitate the thermal removal of trapped guests by heating the material between 80 °C and 150 °C under reduced pressure. This environment effectively evacuates residual water, solvent molecules, and ligand impurities from the porous structure while ensuring the framework remains intact.
By lowering the boiling point of trapped solvents via reduced pressure, the vacuum oven enables deep purification at milder temperatures. This critical step exposes the Ce-MOF's high specific surface area and active sites without risking the structural collapse often associated with high-temperature drying.

The Mechanics of Thermal Activation
Evacuation of Residual Guests
The synthesis of Metal-Organic Frameworks (MOFs) inevitably leaves behind unwanted material within the pores.
Specifically, residual water, solvent molecules, and unreacted ligands often remain trapped after the initial formation of the Ce-MOF.
The vacuum oven provides the necessary thermal energy to volatilize these impurities, driving them out of the intricate pore network.
Lowering Vaporization Thresholds
Under standard atmospheric pressure, removing certain high-boiling-point solvents would require temperatures that might damage the MOF.
The vacuum oven functions by reducing the internal pressure of the system.
This physical change lowers the boiling point of the adsorbed liquids, allowing them to evaporate and escape at significantly lower temperatures (80–150 °C).
Preserving Structural Integrity
Preventing Framework Collapse
Ce-MOF materials depend on a specific crystalline structure to function effectively.
High-heat drying without vacuum support can lead to the collapse of this sensitive framework, effectively destroying the material's utility.
By operating under vacuum, you achieve thorough dehydration and cleaning without subjecting the material to destructive thermal stress.
Unlocking Porosity and Active Sites
The activation process is not merely about drying; it is about functional preparation.
Removing the "clutter" of solvents and ligands exposes the material's high specific surface area.
This creates clear, accessible active sites necessary for downstream applications, such as nanoparticle encapsulation or gas adsorption tasks.
Understanding the Trade-offs
Temperature vs. Time
While vacuum ovens allow for lower temperatures, this can necessitate longer activation times.
If the temperature is set too low (e.g., significantly below 80 °C), you risk incomplete activation, leaving impurities that block pores and skew surface area data.
Conversely, pushing the temperature beyond 150 °C—even under vacuum—risks degrading the organic linkers that hold the Ce-MOF structure together.
Capillary Resistance
Vacuum aids in overcoming the physical forces holding liquids inside the material.
However, in materials with extremely small nanopores, capillary resistance remains a challenge.
Ideally, the vacuum level must be sufficient to overcome this resistance, ensuring that deep-seated moisture is removed rather than just surface-level solvents.
Optimizing the Activation for Your Goals
To ensure the best performance of your synthesized Ce-MOF, tailor your vacuum oven settings to your specific objective.
- If your primary focus is maximizing surface area: Prioritize the removal of all ligand impurities by maintaining the vacuum at the higher end of the safe temperature range (near 150 °C) for an extended duration.
- If your primary focus is structural stability: Operate at the lower temperature threshold (near 80 °C) and rely on a high-quality, deep vacuum to drive evaporation, minimizing thermal stress on the framework.
Correct activation transforms a synthesized powder into a functional, high-performance porous material.
Summary Table:
| Activation Factor | Parameter Range | Impact on Ce-MOF |
|---|---|---|
| Temperature | 80 °C – 150 °C | Facilitates guest removal without framework collapse |
| Pressure | Reduced (Vacuum) | Lowers solvent boiling points & overcomes capillary forces |
| Target Impurities | Water, Solvents, Ligands | Clears pore space to expose active catalytic sites |
| Risk of High Heat | > 150 °C | Risk of organic linker degradation and structural failure |
Elevate Your Material Research with KINTEK
Precision is non-negotiable when activating sensitive materials like Ce-MOFs. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum ovens designed to provide the stable thermal environments and deep vacuum levels required for successful material activation.
Whether you need Muffle, Tube, Rotary, Vacuum, or CVD systems, our laboratory high-temp furnaces are fully customizable to meet your unique research needs. Ensure your frameworks remain intact and your surface areas stay high—contact our experts today to find your solution!
Visual Guide
References
- Simon Lukato, Grzegorz Litwinienko. Enhancing the Green Synthesis of Glycerol Carbonate: Carboxylation of Glycerol with CO2 Catalyzed by Metal Nanoparticles Encapsulated in Cerium Metal–Organic Frameworks. DOI: 10.3390/nano14080650
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- How do miniature vacuum furnaces ensure image stability? Advanced Engineering for High-Resolution Ceramic Imaging
- How does a vertical vacuum furnace handle long or large loads? Optimize Stability and Uniformity for Heavy Components
- What role does a vacuum sintering furnace play in the formation of the 'core-rim' structure in Ti(C,N)-FeCr cermets?
- How does a Grain Boundary Diffusion (GBD) heat treatment furnace improve the performance of high-grade magnets?
- What components make up the vacuum system in a vacuum furnace and what vacuum level can be achieved? Discover the Key Elements for High-Purity Processing
- What is the maximum operating temperature for molybdenum in vacuum furnaces? Key to High-Temp Processing
- How do vacuum furnaces contribute to new material preparation? Unlock Purity and Precision in Synthesis
- What is the primary use of a vacuum hydrogen dual-purpose furnace? Essential for Diamond Synthesis and High-Performance Sintering



















