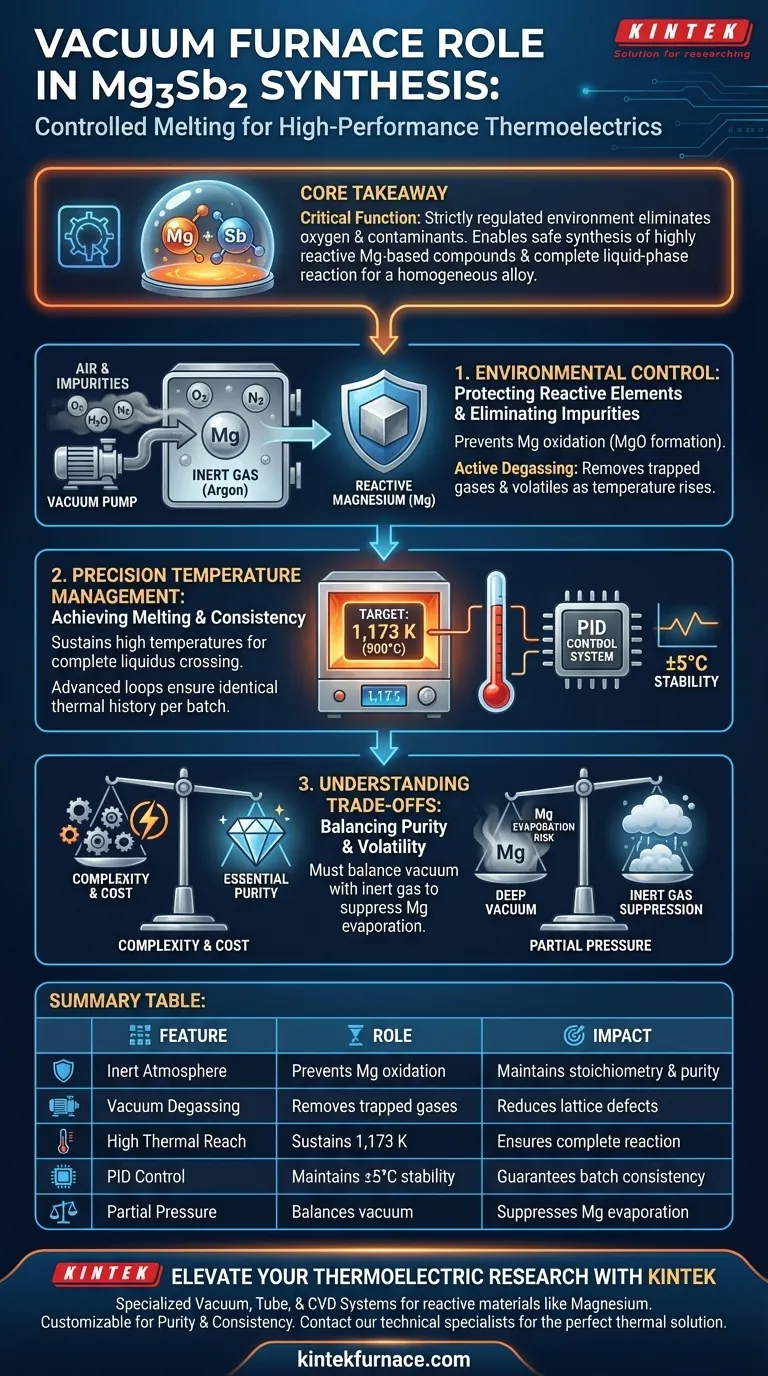
The critical function of a vacuum furnace in the preparation of Mg3Sb2 is to provide a strictly regulated environment that prevents chemical degradation during synthesis. By maintaining a clean vacuum or inert gas atmosphere at high temperatures, the furnace allows magnesium (Mg) and antimony (Sb) to fully melt and chemically react. This isolation is the only reliable method to minimize oxidation and exclude gas impurities, which are detrimental to the material's thermoelectric performance.
Core Takeaway: By eliminating atmospheric oxygen and contaminants, the vacuum furnace facilitates the safe synthesis of highly reactive magnesium-based compounds. It ensures the raw elements undergo a complete liquid-phase reaction to form a homogeneous, high-purity alloy.

The Necessity of Environmental Control
Protecting Highly Reactive Elements
The primary challenge in synthesizing Mg3Sb2 is the high reactivity of magnesium. In a standard atmospheric environment, molten magnesium would rapidly oxidize.
The vacuum furnace solves this by evacuating air and often replacing it with an inert gas. This prevents the formation of magnesium oxide (MgO), ensuring the final material retains the correct stoichiometric ratio.
Facilitating Complete Reactions
To create a high-quality thermoelectric material, the raw elements must bond at a molecular level.
The furnace maintains the necessary conditions for the elements to reach a molten state, allowing them to mix thoroughly. This liquid-phase reaction results in a homogeneous alloy where the Mg and Sb are uniformly distributed.
Eliminating Gas Impurities
Beyond preventing oxidation, the vacuum environment actively degasses the material.
As the temperature rises, trapped gases and volatile impurities within the raw materials are drawn out. This results in a cleaner final compound with fewer defects that could scatter charge carriers and reduce efficiency.
Precision Temperature Management
Achieving the Melting Threshold
The synthesis of Mg3Sb2 requires reaching specific thermal targets, typically around 1,173 K (900°C).
The vacuum furnace is designed to reach and sustain these high temperatures reliably. This ensures the raw materials cross the liquidus line required for complete alloy formation.
Advanced Control Systems
Modern vacuum furnaces utilize sophisticated regulation technologies, such as PID (Proportional-Integral-Derivative) loops and fuzzy logic control.
These systems offer extreme precision, often maintaining temperature stability within ±5℃. This accuracy is vital for ensuring consistency across different batches of material.
Understanding the Trade-offs
Operational Complexity and Cost
While essential for purity, vacuum furnaces represent a significant investment in both capital and operation compared to atmospheric furnaces.
The equipment requires specialized maintenance to ensure seals remain airtight and vacuum pumps function correctly. Furthermore, while modern insulation improves efficiency, the energy required to sustain high temperatures (e.g., 1,173 K) over long reaction cycles is substantial.
Balancing Vacuum vs. Volatility
A "pure" vacuum is not always the perfect solution for magnesium.
Because magnesium has a high vapor pressure, it can evaporate if the vacuum is too deep during the melting phase. Operators must often balance the vacuum with a partial pressure of inert gas (like argon) to suppress evaporation while still keeping oxygen out.
Making the Right Choice for Your Goal
When selecting or operating a furnace for Mg3Sb2 synthesis, consider your specific performance metrics:
- If your primary focus is Material Purity: Prioritize a furnace with a high-integrity vacuum seal and advanced degassing capabilities to minimize oxidation and inclusions.
- If your primary focus is Batch Consistency: Focus on the quality of the thermal control system (PID/Fuzzy logic) to ensure identical thermal histories for every run.
The vacuum furnace is not merely a heating device; it is the fundamental processing tool that bridges the gap between raw, reactive elements and a stable, high-performance thermoelectric compound.
Summary Table:
| Feature | Role in Mg3Sb2 Synthesis | Impact on Material |
|---|---|---|
| Inert Atmosphere | Prevents magnesium oxidation (MgO formation) | Maintains stoichiometry and purity |
| Vacuum Degassing | Removes trapped gases and volatile impurities | Reduces lattice defects and scattering |
| High Thermal Reach | Sustains temperatures up to 1,173 K (900°C) | Ensures complete liquid-phase reaction |
| PID Control | Maintains stability within ±5℃ | Guarantees batch-to-batch consistency |
| Partial Pressure | Balances vacuum with Argon gas | Suppresses magnesium evaporation |
Elevate Your Thermoelectric Research with KINTEK
Precise atmospheric control is the difference between a failed batch and a high-performance alloy. Backed by expert R&D and world-class manufacturing, KINTEK offers specialized Vacuum, Tube, and CVD systems designed to handle reactive materials like magnesium with ease.
Whether you need custom temperature profiles or advanced degassing capabilities, our lab high-temp furnaces are fully customizable to meet your unique synthesis needs. Don't compromise on purity—contact our technical specialists today to find the perfect thermal solution for your laboratory.
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What maintenance is required for vacuum annealing furnaces? Essential Tips for Peak Performance
- How does high-temperature sintering furnace setting influence BaTiO3 microstructure? Optimize Sputtering Performance
- What are the two main designs of vacuum furnaces? Compare Hot Wall vs Cold Wall for Your Lab
- What process conditions does a vacuum furnace provide for Yb:YAG ceramics? Expert Setup for Optical Purity
- What are the benefits of using graphite heating elements in vacuum furnaces? Achieve Extreme Heat and Durability
- How is the furnace body of a vacuum furnace constructed? Explore Its Reinforced, Sealed Design for Extreme Conditions
- What is the significance of an automated pressure control system in a vacuum chamber? Optimize Plasma Nitriding
- Why are vacuum chamber furnaces essential for industrial heat treatment? Ensure Contaminant-Free Precision



















