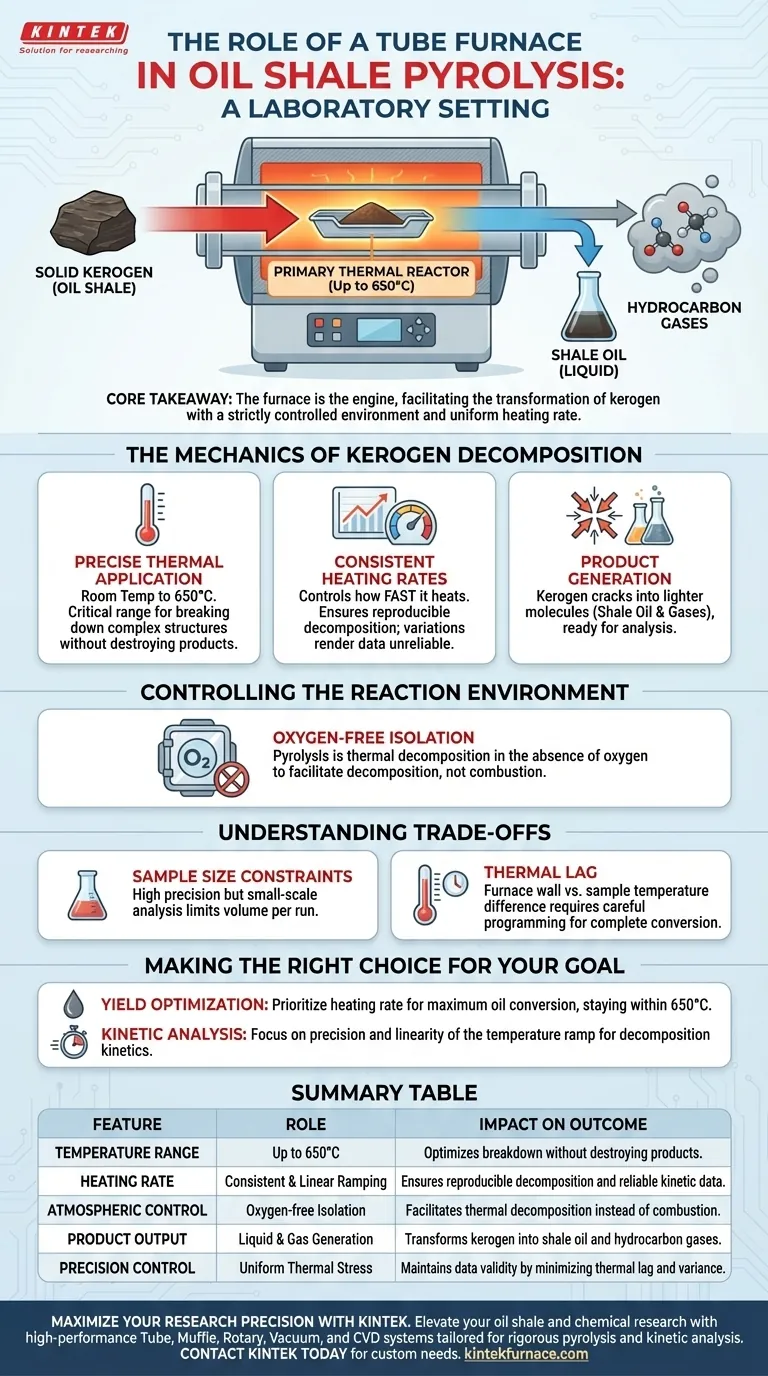
A tube furnace serves as the primary thermal reactor in oil shale experiments, providing the exact conditions necessary to convert solid organic matter into liquid fuel. It functions by heating the sample from room temperature up to 650°C with a consistent heating rate to drive the chemical decomposition of kerogen.
Core Takeaway: The tube furnace is the engine of the pyrolysis process; it facilitates the transformation of kerogen into shale oil and hydrocarbon gases. Its value lies in its ability to maintain a strictly controlled environment and uniform heating rate, ensuring that the resulting data accurately reflects the material's potential.

The Mechanics of Kerogen Decomposition
To understand the role of the furnace, you must understand the material being processed. Oil shale contains kerogen, a solid organic matter that does not release oil until it undergoes specific thermal stress.
Precise Thermal Application
The furnace takes the sample through a rigorous thermal cycle, typically starting at room temperature and ramping up to 650°C.
This specific temperature range is critical because it sits within the optimal window for breaking down the complex organic structures found in shale without destroying the desired products.
Consistent Heating Rates
The primary function of the furnace is not just to reach a temperature, but to control how fast it gets there.
By maintaining a consistent heating rate, the furnace ensures the reproducible chemical decomposition of kerogen. Variations in heating speed can alter the composition of the resulting oil and gas, rendering experimental data unreliable.
Product Generation
As the furnace applies this controlled heat, the kerogen cracks into lighter molecules.
The output of this process is shale oil (liquid) and hydrocarbon gases, which are then collected for further chemical analysis. The furnace acts as the catalyst for this phase change.
Controlling the Reaction Environment
Beyond temperature, the tube furnace dictates the atmospheric conditions of the experiment.
The "Controlled Environment"
Pyrolysis is thermal decomposition in the absence of oxygen.
The tube furnace isolates the sample from the outside air. While the primary mechanism is heat, the enclosed nature of the tube allows the operator to maintain a specific environment that facilitates decomposition rather than combustion (burning).
Understanding the Trade-offs
While tube furnaces are the standard for laboratory pyrolysis, they introduce specific variables that must be managed.
Sample Size Constraints
Tube furnaces in laboratory settings are generally designed for small-scale analysis.
This offers high precision but may limit the volume of shale oil produced in a single run, potentially requiring multiple iterations for extensive product testing.
Thermal Lag
Although the furnace controls the temperature of the heating element precisely, there can be a difference between the furnace wall temperature and the actual sample temperature.
Operators must ensure the "consistent heating rate" programmed into the furnace translates effectively to the core of the oil shale sample to ensure complete kerogen conversion.
Making the Right Choice for Your Goal
The way you utilize the tube furnace depends on the specific data you require from the oil shale.
- If your primary focus is Yield Optimization: Prioritize a heating rate that maximizes the conversion of kerogen to liquid oil rather than gas, staying strictly within the 650°C ceiling.
- If your primary focus is Kinetic Analysis: Focus on the precision of the temperature ramp; the linearity of the heating rate is the most critical factor for calculating decomposition kinetics.
The tube furnace is not merely a heater; it is a precision instrument that dictates the quality, consistency, and validity of your pyrolysis data.
Summary Table:
| Feature | Role in Oil Shale Pyrolysis | Impact on Experimental Outcome |
|---|---|---|
| Temperature Range | Up to 650°C | Optimizes kerogen breakdown without destroying products. |
| Heating Rate | Consistent & Linear Ramping | Ensures reproducible decomposition and reliable kinetic data. |
| Atmospheric Control | Oxygen-free Isolation | Facilitates thermal decomposition (pyrolysis) instead of combustion. |
| Product Output | Liquid & Gas Generation | Transforms solid kerogen into shale oil and hydrocarbon gases. |
| Precision Control | Uniform Thermal Stress | Maintains data validity by minimizing thermal lag and variance. |
Maximize Your Research Precision with KINTEK
Elevate your oil shale and chemical research with KINTEK’s advanced laboratory solutions. Backed by expert R&D and world-class manufacturing, we provide high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems tailored for rigorous pyrolysis and kinetic analysis.
Whether you require precise temperature ramping for yield optimization or customizable high-temp furnaces for unique material testing, KINTEK offers the reliability your lab demands. Don't compromise on your data—Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Yuping Yuan, Zhiyong Chang. Deep Learning Framework for Oil Shale Pyrolysis State Recognition Using Bionic Electronic Nose. DOI: 10.1007/s44196-025-00913-5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- How do multi zone tube furnaces improve laboratory efficiency? Boost Throughput with Parallel Processing
- What happens to quartz tubes in a tube furnace at temperatures above 1000°C? Understanding Devitrification and Material Limits
- Why is a controlled atmosphere tube furnace essential for YBCO? Master Oxygen Stoichiometry for Superconductivity
- Why is a multi-zone tube furnace required for TCVD? Optimize Thermal Management for Precursors
- What are the disadvantages of a tube furnace? Key Limitations for Industrial and Lab Use
- What are the technical requirements for a Tube Furnace in nitrogen-doping? Essential Specs for Metal Oxide Processing
- What role does a high-precision vertical tube furnace play in nickel ore smelting? Master Industrial Smelting Simulation
- What role does a Tube Furnace play in the solution treatment of titanium alloys? Master Material Integrity.



















