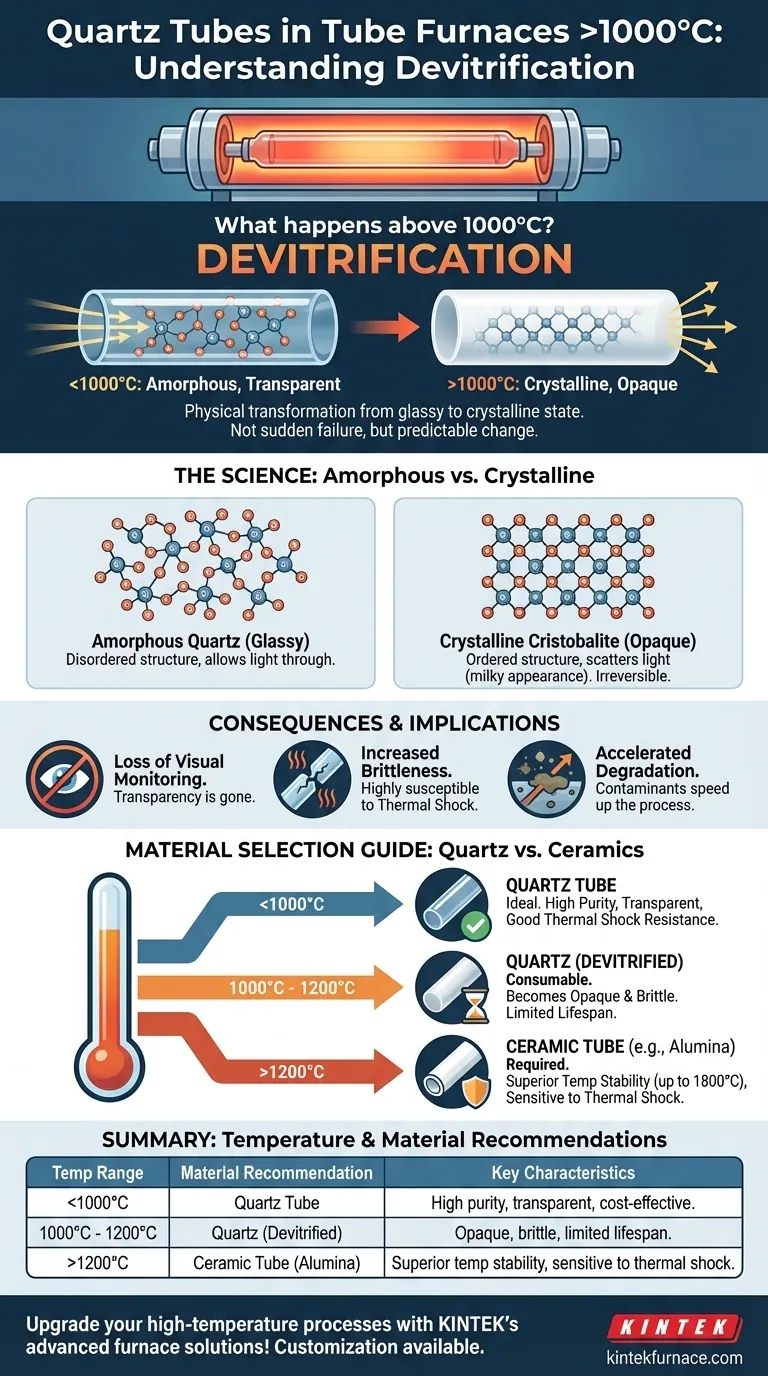
Above 1000°C, a quartz tube undergoes a physical transformation called devitrification. This process causes the heated section of the tube to become opaque or milky-white. This is not a sudden failure but a predictable, inherent characteristic of quartz glass when operated at the upper end of its thermal limit.
The core issue is not that quartz breaks, but that it fundamentally changes. Devitrification is a normal material transition that signals you are operating at the absolute limit of quartz. For any process requiring sustained temperatures above 1200°C, you must move beyond quartz to specialized ceramic materials.
Understanding Devitrification: The Science
What is Devitrification?
Quartz glass is an amorphous material, meaning its silicon and oxygen atoms are in a disordered, random arrangement. This structure is what makes it transparent.
When heated above 1000°C, the atoms gain enough energy to rearrange themselves into a more stable, ordered crystalline structure. This process of changing from a glassy state to a crystalline state is called devitrification.
Why It Becomes Opaque
The new crystalline structure, known as cristobalite, has different optical properties than amorphous quartz.
The boundaries between the newly formed micro-crystals scatter light rather than letting it pass through. This light scattering is what makes the previously transparent tube appear opaque or milky.
Is This a Failure?
Devitrification is considered a normal material change, not a catastrophic failure. The tube does not immediately shatter.
However, the change is irreversible and marks the end of the tube's optimal performance. The crystalline structure is more brittle and less resistant to thermal shock than the original glass.
Practical Implications for Your Process
Loss of Visual Monitoring
The most immediate consequence is the loss of transparency. If your process requires visual observation of the sample inside the tube, an opaque tube renders this impossible.
Increased Brittleness
A devitrified quartz tube is significantly more fragile. It becomes highly susceptible to cracking from thermal shock (rapid heating or cooling) or minor mechanical stress. The risk of tube failure during a subsequent run increases dramatically.
Accelerated Degradation
Devitrification can be accelerated by the presence of surface contaminants, especially alkaline compounds. A perfectly clean tube will resist the process better, but it will not prevent it at these temperatures.
Understanding the Trade-offs
Quartz: Purity vs. Temperature Limit
Quartz is favored in many applications below 1000°C for its high purity, excellent thermal shock resistance, and relatively low cost. Its primary limitation is its firm temperature ceiling, beyond which it begins to degrade.
Ceramics (Alumina): Temperature Resistance vs. Thermal Shock
High-purity ceramic tubes, such as alumina (Al2O3), are the standard for temperatures exceeding 1200°C, with some systems reaching 1800°C.
While they offer superior high-temperature stability, they are generally more sensitive to thermal shock than quartz. They demand slower, more controlled heating and cooling ramps to prevent cracking.
Making the Right Choice for Your Goal
Selecting the correct tube material is critical for the success and reliability of your work. Your process temperature is the deciding factor.
- If your primary focus is on processes below 1000°C: Quartz is the ideal, cost-effective material, offering excellent stability and clarity.
- If you operate between 1000°C and 1200°C: Use quartz with the expectation of devitrification, treating the tube as a consumable with a limited lifespan.
- If your primary focus is on processes above 1200°C: You must use a furnace system designed with a high-temperature ceramic tube, such as alumina.
Matching your material to your thermal requirements is the foundation of safe and repeatable high-temperature work.
Summary Table:
| Temperature Range | Material Recommendation | Key Characteristics |
|---|---|---|
| Below 1000°C | Quartz Tube | High purity, transparent, cost-effective, good thermal shock resistance |
| 1000°C - 1200°C | Quartz Tube (with devitrification) | Becomes opaque, brittle, limited lifespan; treat as consumable |
| Above 1200°C | Ceramic Tube (e.g., Alumina) | Superior temperature stability up to 1800°C, more sensitive to thermal shock |
Upgrade your high-temperature processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable options like Tube Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise solutions for your unique experimental needs, whether you require quartz or ceramic materials. Contact us today to enhance your lab's efficiency and safety!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- How is a Vertical Tube Furnace used for fuel dust ignition studies? Model Industrial Combustion with Precision
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing



















