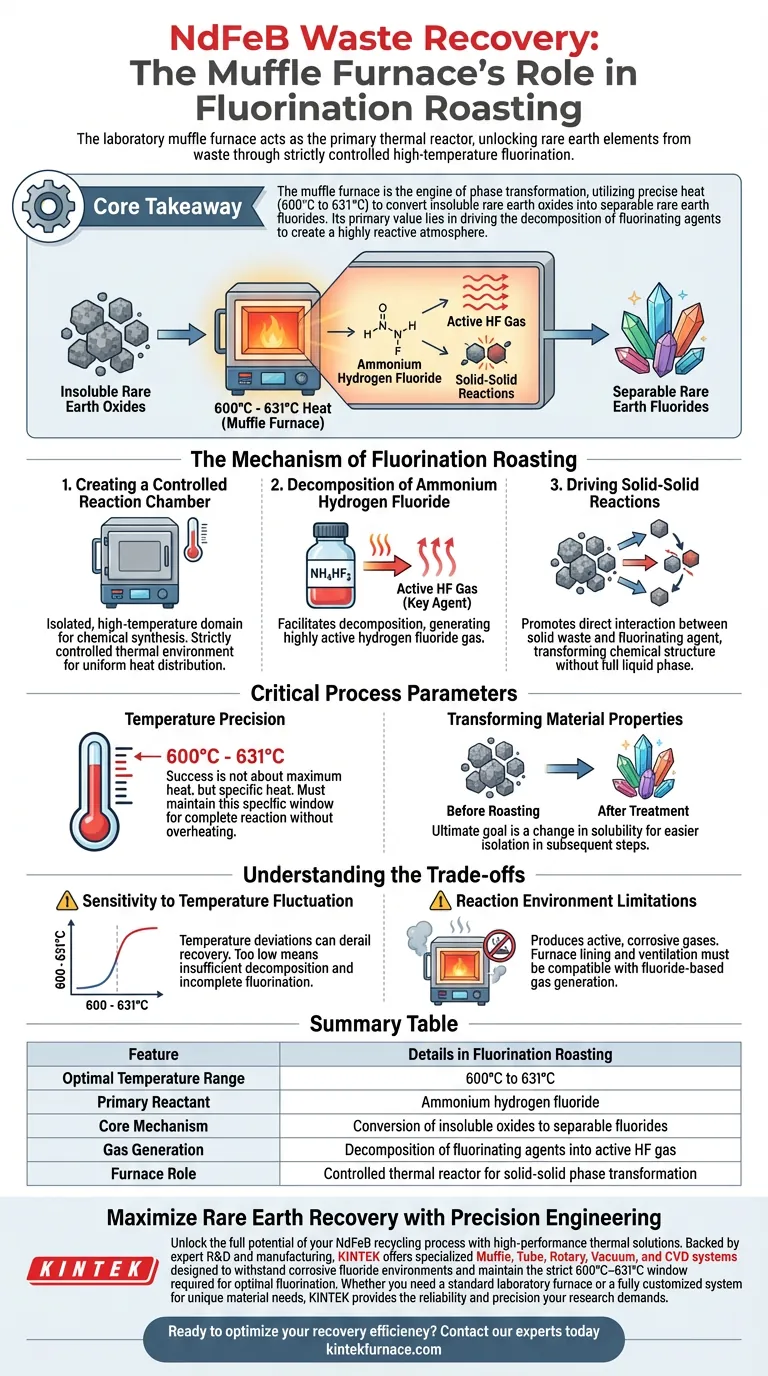
In the recovery of Neodymium Iron Boron (NdFeB) waste, the laboratory muffle furnace acts as the primary thermal reactor for the fluorination roasting stage. It provides a strictly controlled high-temperature environment that forces a reaction between the waste material and ammonium hydrogen fluoride, effectively unlocking rare earth elements for recovery.
Core Takeaway The muffle furnace is the engine of phase transformation in NdFeB recovery, utilizing precise heat (specifically 600°C to 631°C) to convert insoluble rare earth oxides into separable rare earth fluorides. Its primary value lies in driving the decomposition of fluorinating agents to create a highly reactive atmosphere.

The Mechanism of Fluorination Roasting
Creating a Controlled Reaction Chamber
The muffle furnace provides an isolated, high-temperature domain essential for chemical synthesis.
Unlike open-air heating, the muffle furnace maintains a strictly controlled thermal environment. This isolation is critical for containing the reactants and ensuring uniform heat distribution throughout the waste material.
Decomposition of Ammonium Hydrogen Fluoride
The process relies on mixing NdFeB waste with ammonium hydrogen fluoride.
The heat from the muffle furnace facilitates the decomposition of ammonium hydrogen fluoride. This breakdown generates highly active hydrogen fluoride (HF) gas, which is the key agent in the fluorination process.
Driving Solid-Solid Reactions
Beyond gas generation, the thermal energy promotes direct interaction between solid particles.
The furnace drives solid-solid reactions between the waste and the fluorinating agent. This contact transforms the chemical structure of the waste without necessarily requiring a full liquid phase.
Critical Process Parameters
The Importance of Temperature Precision
Success in this stage is not about maximum heat, but specific heat.
The primary reference indicates that the effective range for this reaction is often between 600°C and 631°C. The muffle furnace must maintain this specific window to ensure complete reaction without overheating the materials.
Transforming Material Properties
The ultimate goal of this thermal treatment is a change in solubility.
Before roasting, the rare earth elements exist as insoluble oxides that are difficult to process. After the furnace treatment, they convert into separable rare earth fluorides, which are chemically distinct and easier to isolate in subsequent recovery steps.
Understanding the Trade-offs
Sensitivity to Temperature Fluctuation
The precision of the muffle furnace is a double-edged sword.
Because the process relies on a specific window (e.g., 600–631°C), temperature deviations can derail recovery. If the temperature is too low, the ammonium hydrogen fluoride may not decompose sufficiently to generate the required active HF gas.
Reaction Environment Limitations
While the muffle furnace excels at heating, it is a closed system.
The decomposition produces active gases that are corrosive and necessary for the reaction. Operators must ensure the furnace lining and ventilation are compatible with fluoride-based gas generation to prevent equipment degradation or safety hazards.
Making the Right Choice for Your Goal
To optimize the fluorination roasting stage of NdFeB waste recovery, consider the following:
- If your primary focus is reaction efficiency: Ensure your furnace can hold a steady soak temperature exactly between 600°C and 631°C to maximize the conversion of oxides to fluorides.
- If your primary focus is process consistency: Calibrate the furnace to guarantee uniform decomposition of ammonium hydrogen fluoride throughout the entire batch, avoiding cold spots that lead to incomplete fluorination.
The muffle furnace turns a complex chemical challenge into a manageable thermal process, functioning as the key to unlocking valuable rare earth elements from waste.
Summary Table:
| Feature | Details in Fluorination Roasting |
|---|---|
| Optimal Temperature Range | 600°C to 631°C |
| Primary Reactant | Ammonium hydrogen fluoride |
| Core Mechanism | Conversion of insoluble oxides to separable fluorides |
| Gas Generation | Decomposition of fluorinating agents into active HF gas |
| Furnace Role | Controlled thermal reactor for solid-solid phase transformation |
Maximize Rare Earth Recovery with Precision Engineering
Unlock the full potential of your NdFeB recycling process with high-performance thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems designed to withstand corrosive fluoride environments and maintain the strict 600°C–631°C window required for optimal fluorination.
Whether you need a standard laboratory furnace or a fully customized system for unique material needs, KINTEK provides the reliability and precision your research demands.
Ready to optimize your recovery efficiency? Contact our experts today to find the perfect furnace for your laboratory.
Visual Guide
References
- Optimization of Rare Earth Yield from Fluoride Roasting of Neodymium–Iron–Boron Waste Using Response Surface Methodology. DOI: 10.3390/met15090942
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a muffle furnace ensure the quality of high-temperature solid-state synthesis? Achieve Superior Phase Purity
- How do temperature controllers and electric heating furnaces facilitate different pyrolysis modes in research systems?
- What role does a high-temperature experimental furnace play in sintering Li2Mg3Ti(1-x)ZrxO6 ceramics?
- How should materials be selected for use in a Muffle furnace? Optimize Your High-Temperature Processes
- What type of cooling system is typically used in laboratory muffle furnaces? Discover the Simple Exhaust Design for Safe, Gradual Cooling
- What types of applications are muffle furnaces commonly used for? Essential Uses in Material Analysis, Heat Treatment, and Synthesis
- How does the insulation in a muffle furnace contribute to its efficiency? Unlock Energy Savings and Precision
- Industrial Muffle Furnace Role in MgO Catalyst Preparation: Precision Thermal Engineering for Dry Reforming



















