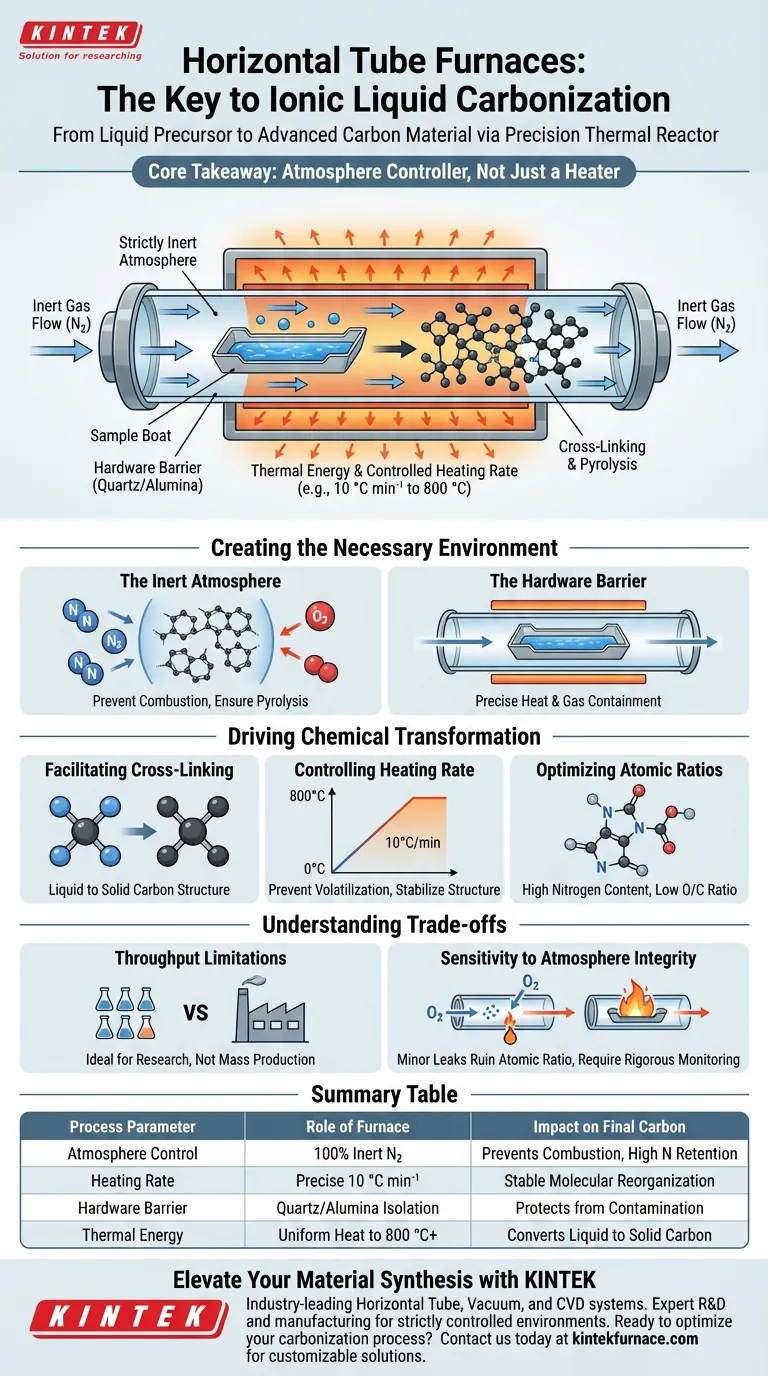
The horizontal tube furnace acts as a precision thermal reactor that converts ionic liquid precursors into advanced carbon materials. It provides a strictly controlled, oxygen-free environment that allows for the pyrolysis and cross-linking of these liquids without causing them to burn away or oxidize.
Core Takeaway The horizontal tube furnace is not merely a heater; it is an atmosphere controller. By coupling a specific heating rate (e.g., 10 °C min⁻¹) with a constant inert gas flow, it forces ionic liquids to undergo chemical cross-linking rather than combustion, preserving high nitrogen content and ensuring the formation of stable carbonaceous intermediates.
Creating the Necessary Reaction Environment
To carbonize an ionic liquid successfully, you must prevent it from reacting with ambient air.
The Role of the Inert Atmosphere
The primary function of the furnace is to maintain a strictly inert atmosphere, typically using a continuous flow of nitrogen.
Ionic liquids are organic salts; if heated in the presence of oxygen, they would simply combust and turn into ash or gas.
The tube furnace ensures that the environment remains completely oxygen-free, forcing the material to decompose thermally (pyrolysis) rather than oxidize.
The Hardware Barrier
The central component is the furnace tube, usually constructed from high-temperature-resistant materials like quartz or alumina.
This tube serves as the physical barrier that isolates the ionic liquid sample from the external environment.
It allows for the precise application of heat while containing the specific gas atmosphere required for the reaction.
Driving Chemical Transformation
Once the atmosphere is secured, the furnace manages the energy required to change the material's state.
Facilitating Cross-Linking
The furnace provides the thermal energy needed to initiate cross-linking reactions within the ionic liquid.
As the temperature rises, the liquid precursors begin to bond effectively, solidifying into a cohesive carbon structure.
This step is the bridge between a raw liquid precursor and a functional solid material.
Controlling the Heating Rate
Precise regulation of the heating rate is critical for determining the final structure of the carbon.
A standard protocol involves a ramp of 10 °C min⁻¹ up to a target temperature of roughly 800 °C.
This controlled ramp prevents rapid volatilization, giving the molecular structure time to stabilize and reorganize.
Optimizing Atomic Ratios
The specific conditions maintained by the furnace directly influence the chemical composition of the final product.
By preventing oxidation, the process yields carbonaceous intermediates with high nitrogen content.
Simultaneously, it ensures low oxygen/carbon ratios, which is often a key performance metric for these materials.
Understanding the Trade-offs
While horizontal tube furnaces offer precision, they introduce specific limitations you must account for.
Throughput Limitations
These furnaces are typically designed for batch processing or small-scale continuous experiments.
They are excellent for research and high-value material synthesis but are generally not suitable for bulk, mass-production throughput without significant scaling modifications.
Sensitivity to Atmosphere Integrity
The success of the carbonization relies entirely on the seal and gas flow integrity of the tube.
Even a minor leak introducing oxygen can ruin the atomic ratio of the ionic liquid derivative, leading to burnout rather than carbonization.
The system requires rigorous monitoring of gas flow rates to maintain the "inert" status throughout the entire heating duration.
Making the Right Choice for Your Goal
When setting up your carbonization process, tailor the furnace parameters to your specific material targets.
- If your primary focus is Elemental Composition: Prioritize a robust gas flow system to ensure the environment is 100% oxygen-free, maximizing nitrogen retention.
- If your primary focus is Structural Integrity: Reduce the heating rate (potentially below 10 °C min⁻¹) to allow for slower, more orderly cross-linking during the phase transition.
The horizontal tube furnace is the gatekeeper that determines whether your ionic liquid becomes high-value carbon or simply evaporates as gas.
Summary Table:
| Process Parameter | Role of Horizontal Tube Furnace | Impact on Final Carbon Material |
|---|---|---|
| Atmosphere Control | Provides 100% inert nitrogen environment | Prevents combustion; ensures high nitrogen retention |
| Heating Rate | Precise 10 °C min⁻¹ ramping | Facilitates stable molecular reorganization/cross-linking |
| Hardware Barrier | High-purity quartz/alumina tube isolation | Protects samples from contamination and ambient oxygen |
| Thermal Energy | Uniform heat application up to 800 °C+ | Converts liquid precursors into solid carbonaceous structures |
Elevate Your Material Synthesis with KINTEK
Don't let oxygen leaks or uneven heating compromise your ionic liquid carbonization. KINTEK provides industry-leading Horizontal Tube, Vacuum, and CVD systems designed for researchers who demand precision. Backed by expert R&D and manufacturing, our furnaces offer the strictly controlled environments necessary to preserve atomic ratios and ensure structural integrity.
Ready to optimize your carbonization process? Contact us today to explore our customizable lab high-temp furnaces and find the perfect fit for your unique research needs.
Visual Guide
References
- Nawaf Albeladi, Robert Mokaya. Ultra-high surface area ionic-liquid-derived carbons that meet both gravimetric and volumetric methane storage targets. DOI: 10.1039/d3ee03957a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- Why is a tube furnace with precise temperature control critical for the preparation of Palladium Borosulfates?
- What environmental conditions does a high-temperature tube furnace simulate for corrosion? Replicate Boiler Realities
- How does a platinum tube heating device assist in studying tungsten work function? Precision Oxygen Purification
- What are the steps for insulation and cooling in a multi zone tube furnace? Master Precise Thermal Control
- Why is high-purity argon gas essential during the pyrolysis of Cu@Zn-NC in a high-temperature tube furnace?
- What role does a Drop Tube Furnace (DTF) play in large-scale wheat straw combustion? Unlock Industrial Performance Data
- Why are controlled atmosphere and vacuum operations important for tube furnaces? Protect Materials and Enable Precision Reactions
- How does a tube furnace contribute to efficient gas recovery? Maximize Gas Capture and Control



















