At its core, controlled atmosphere and vacuum operations in tube furnaces serve two primary functions. They protect materials from unwanted chemical reactions, like oxidation, by removing reactive gases. They also create a highly specific and controlled environment that is necessary to facilitate complex reactions or achieve a material's desired final properties.
The decision to use a vacuum or a specific gas atmosphere is not merely an operational step; it is a fundamental choice that dictates the chemical environment of your process. This control is the deciding factor between producing a high-purity, high-performance material and a contaminated, failed experiment.

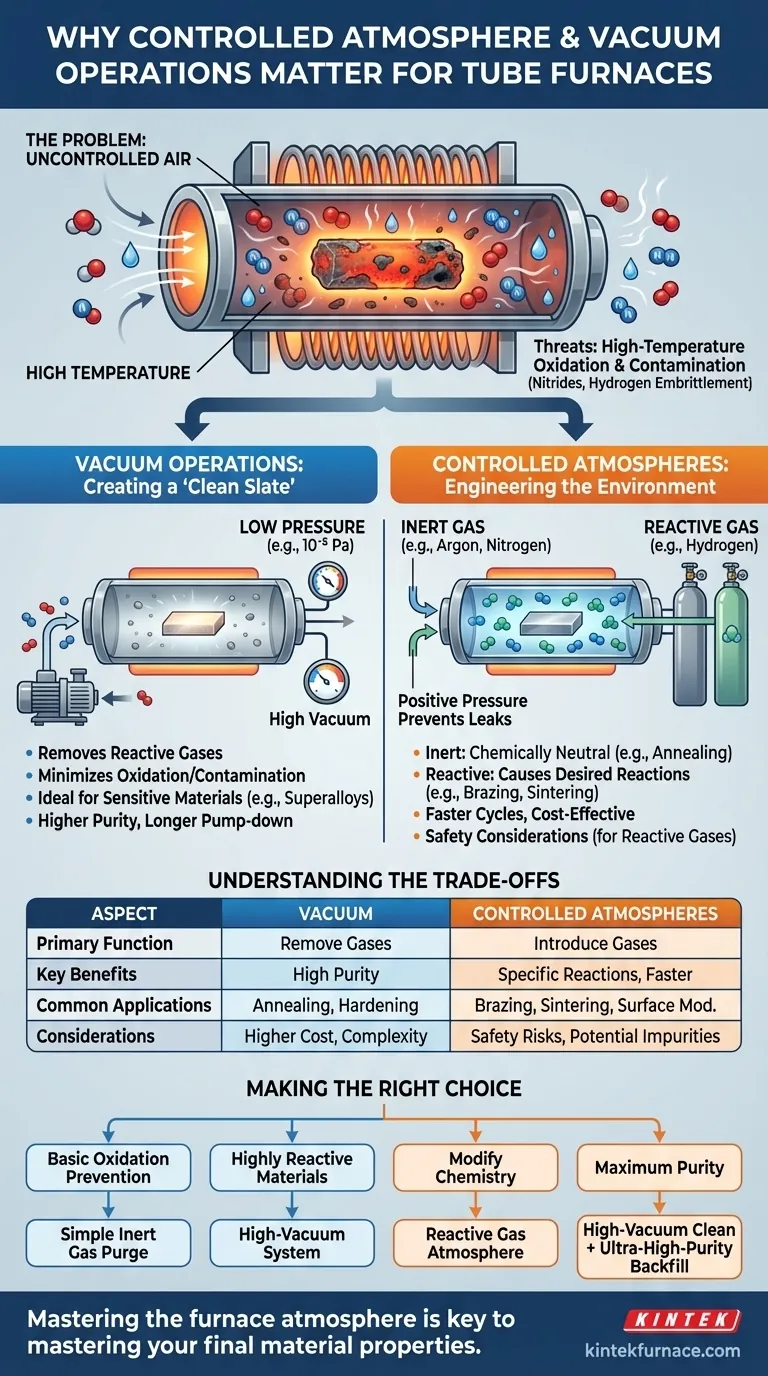
The Fundamental Problem: Uncontrolled Air
At the high temperatures common in tube furnaces, the ambient air we breathe becomes a highly reactive and contaminating agent. Understanding this threat is the first step to appreciating the need for atmospheric control.
The Threat of High-Temperature Oxidation
Oxygen is highly reactive, especially at elevated temperatures. For most metals and many other materials, exposure to oxygen at heat treatment temperatures results in rapid oxidation, forming a layer of oxide scale.
This is not just a surface blemish. This oxidation can alter the material's dimensions, compromise its structural integrity, and ruin its electrical or chemical properties.
Beyond Oxygen: The Risk of Contamination
Air is not just oxygen. It contains nitrogen (~78%), water vapor, and other trace gases.
While nitrogen is often considered inert, it can react with certain metals at high temperatures to form unwanted nitrides. Water vapor can be a source of hydrogen, leading to hydrogen embrittlement in some alloys, or act as an oxidizer itself.
Preserving Material Integrity
Atmospheric control is critical for maintaining the intended composition of your material.
Processes like decarburization, where carbon leaves the surface of steel in the presence of oxygen, can soften the material and degrade its performance. A controlled environment prevents this, ensuring clean surfaces and preserving the material's bulk properties.
How Vacuum and Controlled Atmospheres Solve the Problem
By removing or replacing the ambient air, you take control of the chemical environment. This is achieved in two primary ways: creating a vacuum or introducing a specific, known gas.
Vacuum: Creating a 'Clean Slate'
A vacuum is the most effective way to remove the vast majority of reactive particles from the furnace chamber. In this "air-free" environment, the risk of oxidation and contamination is drastically minimized.
For some processes, like through-hardening or annealing, the vacuum itself is the ideal atmosphere. It provides a clean, neutral environment that prevents any reactions from occurring.
Not All Vacuums Are Equal
The effectiveness of a vacuum is measured by its pressure, or "vacuum degree." A lower pressure means fewer particles and better protection.
A rough vacuum (e.g., 10⁻¹ Pa) may be sufficient for some applications, but processing highly reactive materials like superalloys or advanced ceramics requires a high vacuum (10⁻³ Pa to 10⁻⁵ Pa) to achieve the necessary purity.
Controlled Atmosphere: Engineering the Environment
Sometimes, a process requires a specific gas to be present. This is where controlled atmospheres, often used after pulling an initial vacuum, become essential.
- Inert Atmospheres: The chamber is backfilled with a non-reactive gas like Argon or Nitrogen. This provides a positive pressure environment that physically prevents air from leaking in while remaining chemically neutral.
- Reactive Atmospheres: Specific gases are intentionally introduced to cause a desired chemical reaction. This is fundamental to processes like brazing, sintering, and surface modification, where the atmosphere is an active ingredient in the material's transformation.
Understanding the Trade-offs
Choosing the right atmospheric control involves balancing technical requirements with operational complexity and cost. There is no single "best" solution for all applications.
Vacuum vs. Inert Gas
A high-vacuum system provides the highest level of purity but comes with higher equipment costs, longer cycle times for pump-down, and more complex maintenance.
An inert gas purge is simpler, faster, and less expensive. However, it may not achieve the purity level required for the most sensitive materials, as trace impurities can exist in the gas supply or from incomplete purging.
The Myth of a 'Perfect' Environment
Even in a high-vacuum system, a perfect vacuum is unattainable. A primary source of contamination can be outgassing, where gases trapped within the material itself are released at high temperatures.
This underscores the need for high-quality materials and proper cleaning procedures, as the furnace environment is only one part of the purity equation.
Safety and Process Complexity
Using reactive gases, such as hydrogen for a reducing atmosphere, introduces significant safety considerations. These systems require specialized gas handling equipment, safety interlocks, and ventilation to mitigate risks of fire or explosion.
Making the Right Choice for Your Goal
Your choice of atmospheric control should be dictated directly by your material's sensitivity and your desired outcome.
- If your primary focus is basic oxidation prevention: A simple inert gas purge with Argon or Nitrogen is often sufficient and cost-effective.
- If you are working with highly reactive materials (like titanium or superalloys): A high-vacuum system is non-negotiable to ensure material purity and integrity.
- If you need to actively modify the material's chemistry (e.g., carburizing): A furnace capable of handling specific reactive gases is required.
- If your goal is maximum purity and process repeatability: A high-vacuum cycle to clean the chamber, followed by a backfill of ultra-high-purity inert gas, provides the most controlled environment possible.
Mastering the atmosphere inside your furnace is the key to mastering the properties of your final material.
Summary Table:
| Aspect | Vacuum Operations | Controlled Atmospheres |
|---|---|---|
| Primary Function | Removes reactive gases to minimize oxidation and contamination | Introduces specific gases (e.g., inert or reactive) to control chemical environment |
| Key Benefits | High purity, prevents unwanted reactions, ideal for sensitive materials | Facilitates specific reactions, faster cycles, cost-effective for basic needs |
| Common Applications | Annealing, hardening of reactive metals like superalloys | Brazing, sintering, surface modification with gases like argon or hydrogen |
| Considerations | Higher cost, longer pump-down times, requires high vacuum for purity | Safety risks with reactive gases, potential for trace impurities |
Unlock Precision in Your Laboratory with KINTEK's Advanced Furnace Solutions
Are you struggling with material oxidation or inconsistent results in high-temperature processes? KINTEK specializes in providing tailored high-temperature furnace systems that excel in controlled atmosphere and vacuum operations. Leveraging our exceptional R&D and in-house manufacturing, we offer a comprehensive product line including Tube Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to meet your unique experimental requirements.
Whether you're working with reactive metals, ceramics, or need precise gas environments for brazing and sintering, our solutions ensure high purity, repeatability, and safety. Don't let atmospheric challenges hold back your innovations—contact us today to discuss how we can optimize your processes and deliver reliable performance for your laboratory needs!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide
- How is a Vertical Tube Furnace used for fuel dust ignition studies? Model Industrial Combustion with Precision



















