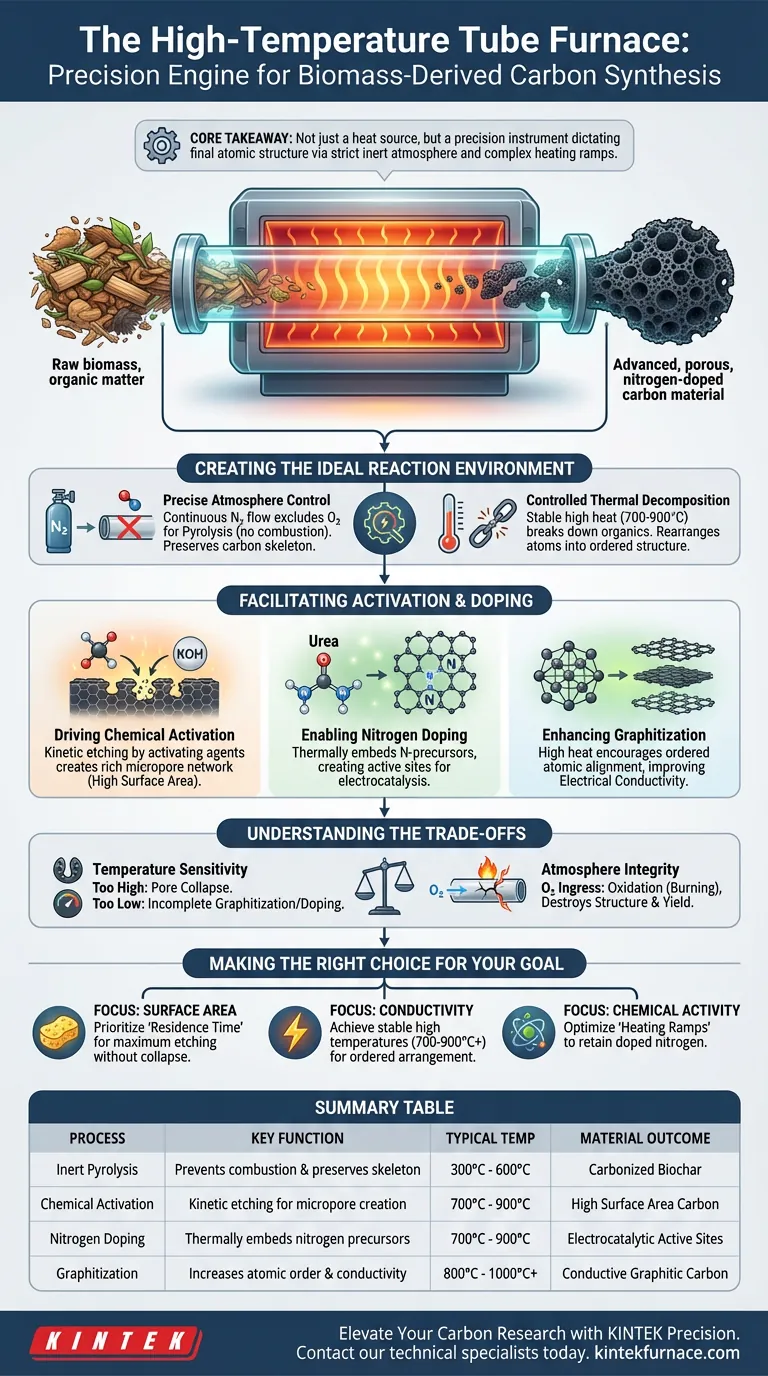
A high-temperature tube furnace functions as the primary reaction vessel that enables the transformation of raw biomass into advanced, nitrogen-doped carbon materials. It provides a strictly controlled thermal environment, typically between 700 °C and 900 °C, while maintaining a steady flow of inert gas like nitrogen. This isolation from oxygen is critical; it allows the biomass to undergo pyrolysis and chemical modification without combusting into ash.
Core Takeaway The tube furnace is not merely a heat source; it is a precision instrument that dictates the material's final atomic structure. Its ability to maintain a strictly inert atmosphere while executing complex heating ramps is the deciding factor in creating micropores and successfully embedding nitrogen atoms from modifiers (like urea) into the carbon framework.

Creating the Ideal Reaction Environment
Precise Atmosphere Control
The most fundamental role of the tube furnace is to exclude oxygen entirely. By maintaining a continuous flow of nitrogen (or occasionally argon), the furnace ensures that the biomass undergoes pyrolysis rather than combustion.
This inert atmosphere is essential for preserving the carbon skeleton. It allows for the safe removal of volatile components during the pre-carbonization stage, leaving behind the fixed carbon required for high-performance applications.
Controlled Thermal Decomposition
Biomass is a complex mix of organic macromolecules that must be broken down systematically. The tube furnace provides the stable high-temperature environment necessary to dehydrate and decarbonize the raw material.
Through precise heating, the furnace induces the thermal decomposition of these organics. This process rearranges carbon atoms, converting amorphous biomass into a more ordered, graphitized structure that serves as the foundation for electrical conductivity.
Facilitating Activation and Doping
Driving Chemical Activation
Chemical activation is a kinetic process where an activating agent (such as KOH) "etches" the carbon surface to create pores. The tube furnace maintains the specific high temperatures required to sustain these reaction kinetics.
By controlling the residence time at these peak temperatures, the furnace allows the activator to react aggressively with the carbon matrix. This reaction creates a rich network of micropores, significantly increasing the specific surface area of the material.
Enabling Nitrogen Doping
For nitrogen-doping, the furnace's role becomes even more critical. It must reach and hold temperatures between 700 °C and 900 °C to facilitate the decomposition of nitrogen precursors like urea.
At these specific thermal energy levels, nitrogen atoms are liberated from the precursor and chemically bonded into the carbon lattice. The furnace’s thermal stability ensures this substitution happens efficiently, creating active sites for electrocatalytic reactions.
Enhancing Graphitization
Beyond porous structure, the electrical properties of the material depend on how the carbon atoms are arranged. The high heat provided by the tube furnace encourages graphitization.
This process aligns the carbon atoms into ordered layers. A higher degree of graphitization, achieved through controlled high-temperature soaking, results in better electrical conductivity for the final product.
Understanding the Trade-offs
Temperature Sensitivity
While high heat is necessary, excessive temperatures can be detrimental. If the furnace temperature exceeds the optimal range for the specific biomass precursor, the pore structure may collapse, reducing surface area.
Conversely, if the temperature is too low, the graphitization will be incomplete. This results in poor conductivity and inefficient nitrogen doping, rendering the material less effective for catalytic applications.
Atmosphere Integrity
The performance of the material is entirely dependent on the purity of the furnace atmosphere. Even minor leaks or interruptions in the nitrogen flow can introduce oxygen.
Oxygen ingress at these temperatures causes immediate oxidation (burning) of the carbon. This destroys the carefully engineered pore structure and significantly reduces the yield of the final product.
Making the Right Choice for Your Goal
To maximize the utility of a high-temperature tube furnace for your specific research or production needs, consider these distinct operational focuses:
- If your primary focus is Surface Area (Porosity): Prioritize precise control over "residence time" at the activation temperature to maximize chemical etching without collapsing the pore structure.
- If your primary focus is Conductivity (Graphitization): Focus on achieving stable temperatures at the higher end of the 700 °C – 900 °C spectrum to ensure ordered arrangement of carbon atoms.
- If your primary focus is Chemical Activity (Doping): Ensure your heating ramps are optimized to retain nitrogen within the lattice, as excessive heat or prolonged exposure can drive the doped nitrogen out of the material.
The precision of your thermal profile is the single most important variable in determining whether your biomass becomes high-value activated carbon or simple charcoal.
Summary Table:
| Process Role | Key Function | Typical Temperature Range | Material Outcome |
|---|---|---|---|
| Inert Pyrolysis | Prevents combustion & preserves carbon skeleton | 300°C - 600°C | Carbonized Biochar |
| Chemical Activation | Kinetic etching for micropore creation | 700°C - 900°C | High Surface Area Carbon |
| Nitrogen Doping | Thermally embeds nitrogen precursors (e.g., urea) | 700°C - 900°C | Electrocatalytic Active Sites |
| Graphitization | Increases atomic order and conductivity | 800°C - 1000°C+ | Conductive Graphitic Carbon |
Elevate Your Carbon Research with KINTEK Precision
Achieving the perfect balance of porosity and graphitization requires absolute thermal control. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed for the rigorous demands of chemical activation and doping. Our lab high-temperature furnaces are fully customizable to your unique research needs, ensuring stable atmospheres and precise heating ramps every time.
Ready to optimize your synthesis? Contact our technical specialists today to find the ideal furnace for your lab.
Visual Guide
References
- Joanna Sreńscek-Nazzal, Beata Michalkiewicz. Chemical Activation of Banana Peel Waste-Derived Biochar Using KOH and Urea for CO2 Capture. DOI: 10.3390/ma17040872
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What type of process environment does a tube furnace provide? Optimized Thermal Solutions for NMA Particle Coating
- How does the design of a dual-zone Tube Furnace facilitate precise metal phosphide conversion? Optimize Heterojunctions
- What is an atmosphere tube furnace? Unlock Precise High-Temperature Processing
- What are the key features of a vacuum tube furnace? Master High-Temp Processing with Precision Control
- How does gas flow impact the performance of a split tube furnace? Optimize Your Process with Precision Control
- What materials are commonly used for the reaction tubes in a tube furnace? Choose the Best for Your Thermal Process
- How does a tube vacuum furnace ensure quality during the solution treatment of aluminum matrix composites? Unlock Precision and Purity for Superior Materials
- Why is precise atmosphere control in a tube furnace critical for Ga2O3 annealing? Optimize Thin Film Defect Engineering



















